A startling new study shows that cells that are exquisitely sensitive to oxytocin are found in a tiny region in the brains of female but not male mice. This could be of great importance in explaining maternal behavior in females and eventually in treating social disorders like postpartum depression and autism spectrum disorder. The study is titled, 'Sexually dimorphic oxytocin receptor-expressing neurons in the preoptic area of the mouse brain', and is published in the journal PLOS One.
A Louisiana State University biologist and his students have discovered a group of cells that are activated by oxytocin in one area of female mouse brains that are not present in the same area in male mouse brains. Image Credit: Ryoichi Teruyama, LSU
Oxytocin is a small molecule produced in the nerve cells of the hypothalamus, but released by the posterior pituitary gland. It is well known for its role in childbirth, when it stimulates uterine contractions, and in lactation, when it triggers milk let-down and enhances further milk production. However, it also plays a very significant part in regulating social behavior, not only in humans but in many species. This includes maternal caring behavior, bonding within a pair and other social settings, anxiety, aggression and fear, as well as sexual behavior.
The current work at Louisiana State University (LSU) Department of Biological Sciences focused on teasing out the essential difference in the oxytocin-receptor expressing neurons in the male brain vs the female.
Oxytocin acts on cells carrying oxytocin receptors (OXTR) within the brain to trigger social behavior. Oxytocin-responsive cells are known to be present in the medial preoptic area (MPOA) of the female brain, an area that is currently thought of as facilitating maternal behavior. Collections of nerve cells called nuclei have been found in this area, and are known to be different in males and females.
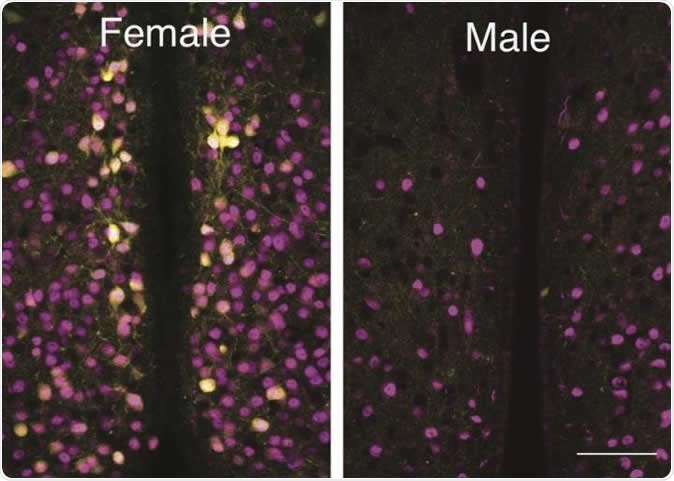
The number of cells bearing OXTR in the MPOA was studied using a modified yellow fluorescent protein to pick out these cells among other neurons. The gene expressing this dye, called Venus, was inserted into a breed of laboratory mice to exactly replace the OXTR gene at its natural locus, in order to achieve a natural pattern of expression of both genes in the heterozygous cells. These mice were used to examine the distribution of OXTR cells in male and female mice. The use of the OXTR-Venus mice allowed precise localization of these cells, unlike earlier methods like autoradiography and in situ hybridization of OXTR mRNA, which are relatively insensitive in detecting differences in such a small area as the AVPV.
The number of OXTR-Venus cells was significantly higher in females compared to males throughout the medial part of the preoptic area (MPOA). However, in one small part called the anteroventral periventricular nucleus (AVPV) these cells were present only in females. Though sex-specific differences in OXTR-Venus cells do occur in other parts of the brain, the current study showed for the first time the exclusively female expression of OXTR within the AVPV.
Overall, the AVPV in females had an average of 615 ± 43 OXTR-Venus cells whereas males had 14 ± 2 of these cells. In contrast, the total number of such cells in the rest of the MPOA was more or less similar in both sexes, showing that the difference in cell number was largely localized to the AVPV. This is a highly significant indicator of how strongly these cells are involved in regulating maternal behavior.
In the second stage of the current study, the researchers looked at whether the female expression of OXTR cells in the AVPV was due to the presence of estrogen in females. They found that estrogen receptors of the type ER α were found on these cells in the AVPV in females. When the ovaries were removed, these OXTR-Venus cells were no longer expressed in the AVPV, but continued to be found in other brain areas. Estrogen supplementation for two weeks increased their number within the AVPV, though not to earlier levels.
In summary, OXTR expression in the AVPV occurs chiefly in females and in response to estrogen stimulation, which occurs after childbirth. This suggests that these OXTR neurons are involved in regulating maternal physiology and behavior. By way of extrapolation, a change in the pattern of oxytocin receptors could affect the way females respond to childbirth, by inducing postpartum depression.
Postpartum depression is a mental health condition affecting about 10% to 20% of mothers after childbirth, which reduces the quality of life in mothers and impacts child development as well, by interfering with normal maternal-child bonding and interaction, and hindering child care. Children born to such mothers show an increased risk for many types of medical problems as well as intellectual, emotional and behavioral issues. The discovery of sex-specific differences in the distribution of cells that respond to this hormone which directly induces maternal behavior could help unlock new therapies for this condition.