Please give an overview of the recent compliance pressures that have been put on pharmaceutical companies.
Compliance always has always been a high priority for data integrity in the pharmaceutical industry, along with a relatively new pressure to convert to electronic records. Many pharma companies still use "hybrid systems" combining paper and electronic records: an electronic instrument is used to print results on paper.
E.g. in WHO Annex 5: "Guidance on good data and record management practices", Appendix: the WHO states, "The use of hybrid systems is discouraged" and later they state, "Replacement of hybrid systems should be a priority". Clear statements that increase the pressure for going electronic and ensuring a high level of data integrity in all areas of the pharmaceutical industry.
What are the hurdles and limitations of digitizing a formerly paper-based set of records and procedures?
Typically, a pharmaceutical analytical QC laboratory has a large mixture of instruments that generate data and results. Different techniques, brands and models. Many of them might not come with software and even if they do, it is a big effort to validate them all. A proper computer system validation may take 3-6 months depending on the level of experience of the validation team and if they follow certain practices.
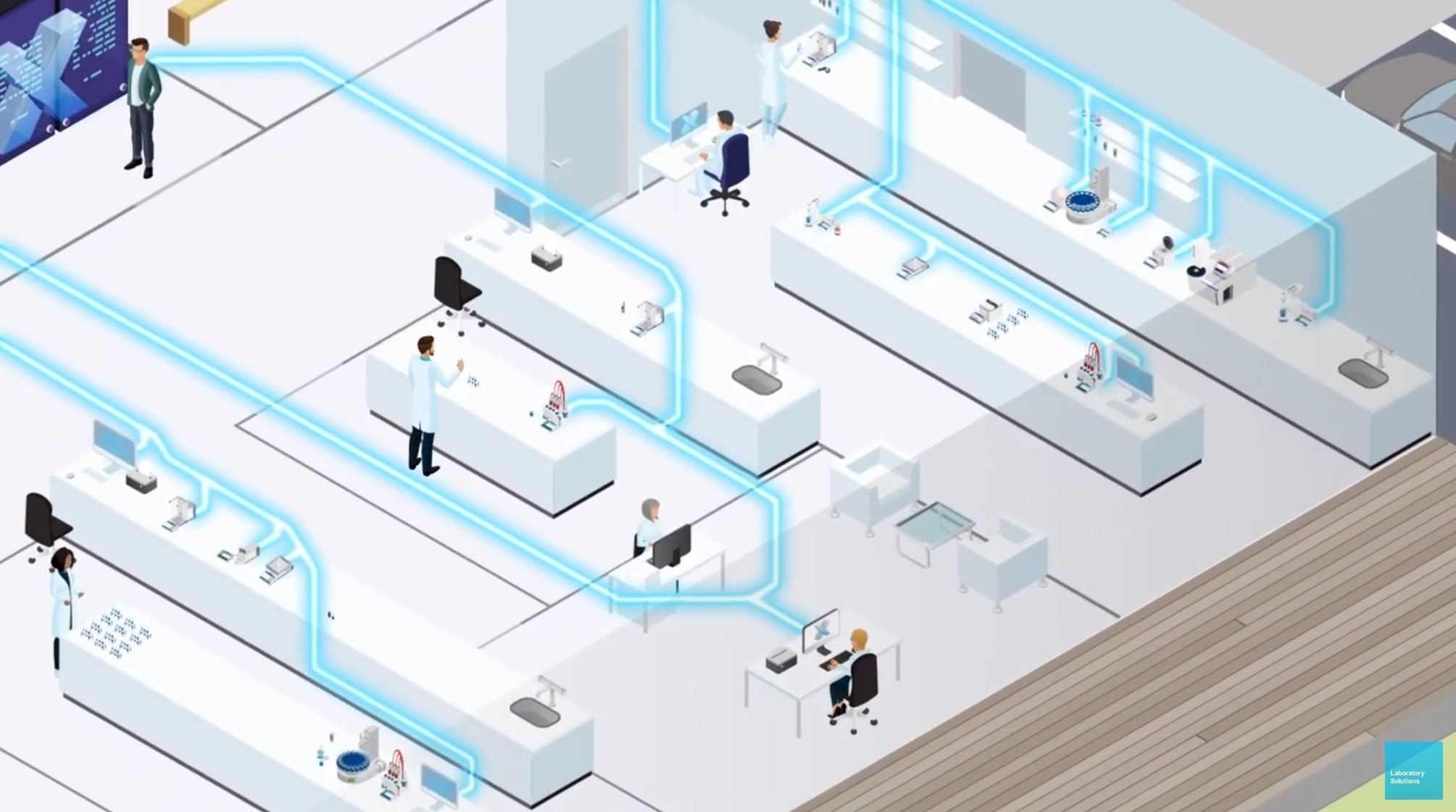
There are companies who offer the integration of all instruments, e.g. via signals from the printer port. These are individual work-arounds and often transport insufficient information. In order to be compliant, it often takes more than is provided, especially meta data. Validation of those systems can be a bit of a pain as individual risk assessments of each connection must be considered.
"Off the shelf" software solutions should be preferred here, as they typically are better tested and maintained. Suppliers can offer standard support and prepare documentation that make the validation and maintenance a lot easier. Software platforms that connect multiple instruments to common standard software make this validation even more easy and valuable.

In the past, some years ago, we heard a lot that the IT department will not allow sending of results into the company's database. This has changed now. Seamless data flows are at the core of digitalisation projects. Manual transcription will be the exception in future.
A few instruments that have no electronic connectivity will always exist and they will always create paper records. However, I would expect that the pressure on these systems on following the ALCOA rules rather will increase for strong data integrity in the pharmaceutical industry.
When changing or upgrading to a new instrument what considerations should be taken with regards to data integrity, compliance and user management?
The selection process of instruments is critical. As a crucial part of the computer system validation process it has to be defined what the system technically should be able to do. When selecting a system I would recommend looking for platform solutions, off the shelf.
A platform solution can connect multiple instruments and centrally manage methods, users and results. The common elements are easier to validate as you start with the platform and add instruments as an amendment via change control. This is cost effective, as the platform validation has to be done only once for a number of instruments.
When is a good time to change software platforms, and why?
Replacing an existing validated installation without need is not recommended. However, systems may have reached the end of lifetime, are no longer supported and are replaced by a successor.
This is a good time to evaluate alternatives. With platform solutions I would say as a general rule is: the more instruments that can be connected, the bigger the business benefit, the lower the effort for computer system validation in the laboratory.
Does an instrument need to be connected to a PC for it to need to be compliant?
This is what the regulation agencies (FDA, MHRA, WHO…) recommend – for good reasons. However, companies often claim their "software or instrument is compliant". Theoretically, many scenarios can be made complaint. This includes electronic records on instruments without a PC. Actually leaving out the PC does not simplify your setup although it might appear in the first moment. When looking at the total cost of ownership it is not even cheaper.
The compliance risks are quite high if you leave out the PC. You risk losing data and at some stages it can be really inconvenient and cumbersome, e.g. if the instrument is the only qualified reader of your data and when technical problems occur or the instrument is retired.
Instrument/software suppliers can only support compliance by technical controls. In order to be compliant, it also takes procedural controls and administrative controls, which is done by the people in the laboratory of the pharma company. If they do it badly, you are not compliant.
If you collect your results, raw data, methods and user-management… on an external PC it is much easier to handle compliance risks, eliminate most of the risky by technical controls. Separating the data from the instrument is recommended for good reasons.
It's all about risks: Looking into FDA warning letters of the last 5 years where concepts of electronic records on instruments failed underline the manifold risks of this concept.
What does Mettler Toledo offer within its range of instruments and software solutions to meet these high standards?
Mettler Toledo offers a software called LabX. This software platform potentially connects around 40 % of your QC lab instruments, including multiple balances, titrators, Karl-Fischer, pH, digital density, refractometry, UV/VIS Spectrometry and melting point.
How can I speed up my lab analyses with LabX software?
LabX comes with an optional server version to connect many instruments in a network. It has an optional "compliance package" that supports technical controls of the requirements. We offer support/service for instrument qualification (IQ/OQ) and support important steps of your computer system validation.
What features should quality officers look for in computer system validation?
Obviously, the most important feature is that the software is capable of proper performance (fit for purpose). It has to generate valid results. It is part of the selection process that you check and confirm that the instrument is capable of fulfilling the requirements.
A bit less obvious are the support capabilities of the supplier. Can they offer training, which is important for the CSV process? How is the documentation support? Does the supplier offer IQ/OQ services? How does the supplier handle updates? Are those properly documented? Is the updates supervised or audited by an external expert?
How to fulfill regulatory needs of my lab in an easy way?
How could we simplify our computer system validation processes? How can we get support?
If you read the latest revisions of USP 1058 or GAMP5 you will see the keyword "Risk based approach". This means validation effort according to a risk, a system comprises. Instead of always applying the maximum effort, you may skip some documents or procedures, simplify, and use less effort, time and money. USP 1058 and GAMP5 give you guidance how to do that.
In order to speed up the CSV process, use as much help as you can: from your supplier, from your own QA department and depending of your level of experience, use a well-versed external consultant, but use the consultant to learn in order to be able to defend your process/documents with confidence in an audit.
Resource the project adequately. Get enough people in your organization involved. Use your suppliers QMS, get enough training of the application.
What is the future for data integrity and compliance in the pharmaceutical industry? What role will Mettler Toledo play?
The future of data integrity is digital. It does not take much to predict this. Whereas paper records will always be accepted, especially when there is no electronic alternative, it will become more and more difficult to pass audits with paper, I suppose.
More and more instrument suppliers will offer software platforms with the capability of connecting most of their instruments. Synergies of connecting multiple instruments have so many business benefits for our customers that this trend will rather grow.
Is there a simple solution for my lab software systems?
Third party integrators will be needed for those instruments that do not come with software. However, this will be the exception as this solution is more complex and cumbersome to make, maintain, document and validate. Thus, whenever possible, "off the shelf" solutions from instrument suppliers most likely will be preferred. Instrument suppliers have full control of their parameters, will be the first who know about firmware and functionality changes and perform intensive testing with the changes.
Mettler Toledo has been very successful with LabX and the related instrument portfolio. We invented some really innovative process improvements that our customers appreciate and gave us advantages in the market. Our R&D department will continue to develop this path.
Where can readers find more information?
- C. Burgess and R.D. McDowall, An integrated Risk Assessment for Analytical Instruments and Computerized Laboratory Systems, Spectroscopy 28(11)1, (www.spectroscopyonline.com), 2013
- Lorrie Vuolo-Schuessler, Karl E. Newton, Paul Smith, Christopher Burgess, R.D. McDowall, Harmonizing USP <1058> and GAMP for Analytical Instrument Qualification, PHARACEUTICAL Engineering, Vol 34, No1, January/February 2014 (https://pdfs.semanticscholar.org/cd8c/33ee2db998c16a535cadd6e4b23bdcad58e7.pdf)
- Robert D. McDowall, Christoph Jansen, Cost-effective computer system validation for assuring e-records compliance ("how to avoid death by compliance"), webinar, (https://www.mt.com/us/en/home/events/) 2019
About Christoph Jansen Ph.D
Christoph Jansen Ph.D. works as a technical key account manager for the Mettler Toledo Analytical Chemistry (AnaChem) division. His background is Analytical Chemistry and he is specialized on systematic laboratory improvements.
He supports customers around the globe and gives presentations about lean laboratory, data integrity, general concepts of Computer System Validation as well as topics around the future of the laboratory: Lab 4.0, digital transformation or IoT in the lab.