Researchers at the Icahn School of Medicine, Mount Sinai, New York have reported a pilot clinical trial with a “nanoparticle-based photothermal cancer therapy” for prostate cancers. Their work with this targeted therapy is published in a study titled, “Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study,” in the latest issue of the journal Proceedings of National Academy of Science.
Art Rastinehad, DO, Associated Professor of Urology and Radiology at the Icahn School of Medicine at Mount Sinai, is leading the first clinical trial of gold nanoparticles to treat prostate cancer.
They write that prostate cancers are one of the commonest non-skin cancers in the United States and affects around 10 percent of all men in their lifetime. The team explained that the prostate cancer usually lie close to several important and vital lower abdominal structures such as the urethra, nerves and blood vessels and treating the cancer becomes difficult without injuring or harming these adjacent structures.
The team devised these Gold-silica nanoparticles that were designed to absorb “near-infrared light at wavelengths of high tissue transparency”. These nanoparticles were then used to provided localized treatment to the prostate cancer. They explained that this targeted therapy helped save the adjacent organs and also reduced the treatment related side effects. The authors of the study write introducing the gold nanoparticles, “Gold nanoparticles absorb light intensely, giving rise to the vivid optical coloration of stained glass windows popularized during medieval times, a property now known as collective electronic excitation or plasmon resonance.”
Gold Nanoparticles
They explained, “...hollow gold nanoparticles could substantially shift resonances to longer wavelengths than solid gold nanoparticles, with a resonant frequency controlled by the spherical shell dimensions.” They wrote that near-infrared nanomedicine could be used in, “probe-based imaging of tumor margin”, “remotely triggerable drug or gene delivery” etc. The most promising field where this technology could be used, they write is the “targeted photothermal cancer therapy.” They called the gold-silica nanoparticles or GSNs Auroshells.
The team wrote that these “biocompatible gold nanoparticles”. Have a special ability to absorb the light at near-infrared wavelength and turn it into heat. This localized generation of the heat helps in killing the targeted tumour cells and ultimately reduced the tumour without affecting the healthy cells. The study showed that use of these nanoparticles helped prolong the remission of the cancer patients when used alongside “magnetic resonance–ultrasound fusion imaging” that was used to locally burn away the “low-intermediate-grade tumors within the prostate.”
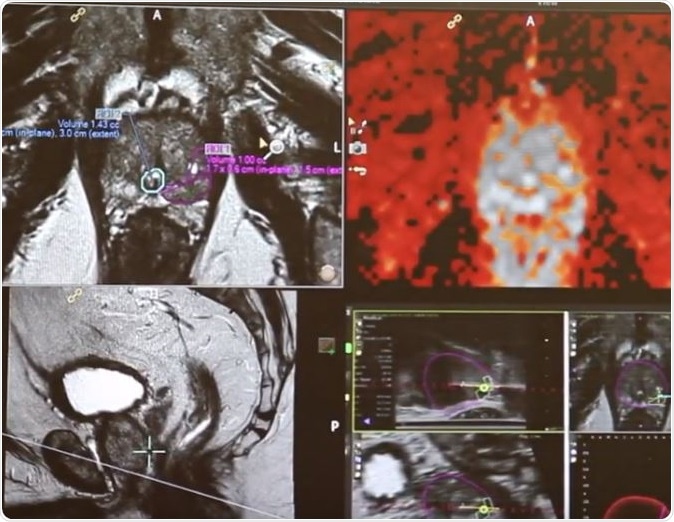
The researchers wrote, “AuroLase Therapy (Nanospectra Biosciences, Inc., Houston, TX) is a focal ablation modality that relies on laser excitation of GSN to selectively target and treat focal lesions within the prostate.” In this therapy the particles collect in the solid tumor tissue where they can act. The therapy based technology is devised by Rice University by engineer and chemist Naomi Halas, PhD, and Duke University bioengineer Jennifer West, PhD. Lead author Ardeshir Rastinehad, DO, Associate Professor of Urology, and Radiology, at the Icahn School of Medicine at Mount Sinai went ahead to devise the clinical therapy that could be used in the pilot study.
For this study the team included 16 men (aged between 58 and 79 years) with diagnosis of “low- or intermediate-risk localized prostate cancer.” They were all given intravenous infusions of gold silica nanoparticles and “high-precision laser ablation”, along with “multiparametric MRI of the prostate at 48 to 72 hours.” For these patients ultrasound guided biopsies were taken from the tumours at 3 and 12 months after the therapy. Along with these targeted fusion biopsies, the patients also underwent 12 core systematic biopsy at 12 months after the therapy.
Results revealed that patients on gold silica nanoparticles showed better local ablation of the tumour (in 94 percent cases or 15 of the 16 patients). Among all the men treated, “International Prostate Symptom Score or Sexual Health Inventory for Men” scores were collected and results showed that the scores were similar in all men receiving the treatment. Overall the treatment protocol was found to be safe and feasible for men presenting with low or intermediate risk localized cancer of the prostate or cancer that has not spread to other organs. This treatment did not affect the genitourinary system of the patients and there were no serious complications.
Dr. Rastinehad said in a statement, “Gold-silica nanoshells infusion allows for a focused therapy that treats the cancer, while sparing the rest of the prostate, thus preserving a patient's quality of life by reducing unwanted side effects, which could include erectile dysfunction and/or the leakage of urine.”
Authors wrote in conclusion, “This current pilot device study demonstrates that GSN-directed laser excitation and ablation is a safe and technically feasible procedure for the targeted destruction of prostate tumors.” Dr. Ash Tewari, Chair of the Department of Urology at the Mount Sinai Health System and the Kyung Hyun Kim, MD Professor of Urology at the Icahn School of Medicine at Mount Sinai, in a statement said, “Mount Sinai's interventional urology program is research-driven and offers patients minimally invasive treatment therapies that improve quality of life. Dr. Rastinehad's gold nanoparticle research shows that patients are not only benefiting from this treatment, but also experiencing minimal side effects.”
Journal reference:
Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study, Ardeshir R. Rastinehad, Harry Anastos, Ethan Wajswol, Jared S. Winoker, John P. Sfakianos, Sai K. Doppalapudi, Michael R. Carrick, Cynthia J. Knauer, Bachir Taouli, Sara C. Lewis, Ashutosh K. Tewari, Jon A. Schwartz, Steven E. Canfield, Arvin K. George, Jennifer L. West, Naomi J. Halas, Proceedings of the National Academy of Sciences Aug 2019, 201906929; DOI: 10.1073/pnas.1906929116, https://www.pnas.org/content/early/2019/08/20/1906929116