New research suggests that the problem of bone loss caused by microgravity conditions in space flight may be successfully treated using the time-tested drug melatonin. Melatonin increases the level of calcitonin in bone, which suppresses the bone-destroying activity of osteoclastic cells and helps maintain bone density.
Bone mass depends on the stable balance between the bone resorption carried out by bone osteoclasts and the bone mass building achieved by osteoblasts. It has been known for a long time that staying in space leads to a significant reduction in bone density. This is a problem which can prevent long-term stays in space stations, for instance. The detailed mechanism is not known, which hampers the development of more effective therapies. The first step to understand this was to find a suitable model of bone metabolism in space, since conventional in vivo cell cultures obviously won’t work in outer space conditions.
The study, published in the Journal of Pineal Research, uses fish scales to study bone metabolism and explains how this pathway works.
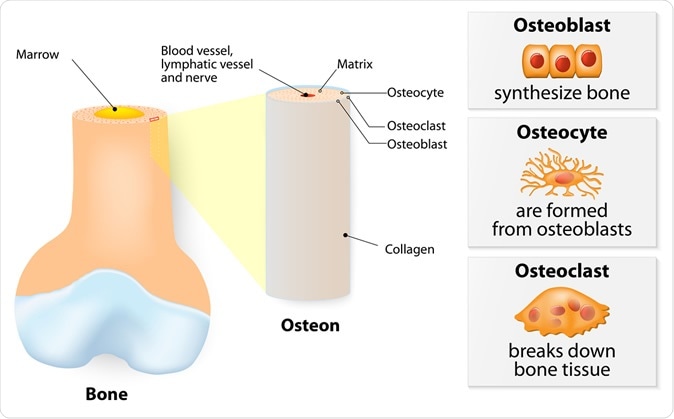
Internal structure of a bone. 3 types of cells are found within bone tissue: Osteoblasts, Osteocytes, and Osteoclasts - Illustration Credit: Designua / Shutterstock
What do we know?
Osteoclasts activate a molecule called RANK on their nuclei which in turn is a receptor for the RANK ligand (RANKL), found on osteoblasts as well as stromal cells. This binding is essential for the production and activation of osteoclasts. Thus osteoclasts and osteoblasts are intimately related in their lifecycle. The molecule called osteoprotogerin (OPG) is also produced by stromal cells and inhibits RANKL, limiting osteoclast production. The ratio of RANKL to OPG is very important in directing bone metabolism. It is therefore essential to study the osteoclasts in tandem with stromal cells to understand how bone loss occurs in space flight.
The current experiment uses regenerating teleost (goldfish) scales, which are calcified tissues strongly resembling membranous bones in mammals. They have hydroxyapatite crystals as well as the two layers of the bone matrix, an outer osseous and an inner fibrillary layer. Following scale removal, regenerating scales contain higher numbers of both osteoclasts and osteoblasts. Scales are also a good choice for this experiment since they act like mammalian bones when exposed to gravity and hormones. In outer space, they can be cultured in the same medium for 10 days at 4o C and are readily available.
In nature, osteoclasts help, among other functions, to help release calcium from the bones for egg production during the spawning season.
The researchers focused on the role of calcitonin, a hormone that inhibits osteoclastic activity, in goldfish, in bone resorption. They also looked at melatonin.
Melatonin is the chief product of the pineal gland, and is produced in the dark, thus showing a detectable circadian rhythm. Earlier research by the same investigators in 2002 showed its ability to suppress osteoclast activity in goldfish scales in culture. Other studies have shown that removal of the pineal gland causes deformed spines in a variety of species, and the bone-strengthening effect of melatonin in women after menopause. Recent research suggests that melatonin could have multiple functions in the body because it was found to be secreted by a wide variety of tissues, including the ovary, skin and intestine, apart from the brain and retina.
How was the study done?
Regenerating goldfish scales were examined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The scales were packed with and without melatonin in culture chambers and 96-well plates, and packed in the space shuttle Atlantis to the Japanese space laboratory on the International Space Station (ISS). One was frozen to enable enzyme activity to be studied. The others were first stored at 4o C before incubating them at 22o C for 86 hours under two sets of conditions – in-flight microgravity or in-flight artificial gravity. They were then processed for further enzyme and morphological analysis.
What did the study show?
The regenerating scales showed networks of grooves in those regions that had enlarged foci. Evidence f osteoclastic activity was seen along the groove edges, in the form of higher levels of the osteoclastic enzyme tartrate‐resistant acid phosphatase (TRAP), in microgravity and artificial gravity conditions. Osteoclasts appeared larger and the average number of nuclei among multinucleated osteoclasts was increased in microgravity scales compared to those grown in artificial gravity. Again, there were more multinucleated osteoclasts with actin rings, and the rings themselves were larger. The rings were found along the grooves of the scales, which showed increased groove-related osteoclastic activity. Thus the grooves became wider as the bone along the groove walls was lost. However, there was no significant difference in the overall number of nuclei when all osteoclasts were studied together.
.jpg)
(a) A goldfish and a binocular view of an ontogenic scale. Grooves radiate from the center focus. (b) Binocular views of a regenerating scale on day 14 (left) and the same scale stained for TRAP activity, indicated by the red color along the grooves (right). The grooves formed a mesh-like structure at the center of the goldfish scales. (c) Light microscopic views of the surface on the osseous layer of the regenerating goldfish scales cultured for 86 h at 22°C on the ground. TRAP staining (left) and F-actin staining (right) superimposed with DAPI staining of the nuclei. A TRAP-positive multinucleated osteoclast possessed an actin ring along the groove. (d) Schematic illustration of morphological features of goldfish scales. Fish scales can regenerate following scale removal. On the groove edge of the goldfish scales, a multinucleated osteoclast exhibited a well-developed RB and CZs (e). Active cuboidal osteoblasts (f) are observed at the periphery and on the dermis side of the scale. They represent a highly-developed rER and Go. OB, osteoblast; OC, osteoclast; TRAP, tartrate-resistant acid phosphatase; DAPI, 4', 6-diamidino-2-phenylindole; N, nucleus; RB, ruffled border; CZ, clear zone; rER, rough endoplasmic reticulum; Go, Golgi apparatus. Scale bars = 1 mm in a and b, 10 μm in c, 5 μm in e and f. Image Credit: Kanazawa University
Melatonin levels in the regenerating scales were at higher levels than in the ontogenic scales. However, genes associated with melatonin-synthesis, such as acetylserotonin O-methyltransferase, were expressed at lower levels in microgravity and artificial gravity conditions, compared to the ground.
Proteins which stimulated bone resorption such as matrix metalloproteinase‐9 (MMP‐9), cathepsin K (Ctsk) and TRAP all increased in microgravity conditions compared to artificial gravity. The RANKL:OPG ratio increased significantly due to increased RANKL and decreased OPG production, favoring osteoclast activity. Cyclo-oxygenase-2α (COX-2α) also increased, and this could further increase RANKL levels since this also promotes bone resorption. However, calcitonin expression decreased in microgravity, which means loss of inhibitory control over osteoclastic activity.
Melatonin helped restore the expression of all these genes to more normal levels, comparable to those in artificial gravity. It reduced osteoclastic activity and increased osteoclastic inhibition, as well as reducing the level of RANK, an osteoclast biomarker. Melatonin may regulate osteoclast RANK expression and osteoblast RANKL expression to suppress osteoclastic activity, as well as increase OPG levels.
At the same time, osteoblastic cellular signals related to osteoblasts were increased in microgravity compared to artificial gravity, though only one attained significant levels (osterix). These were restored to artificial gravity levels by melatonin treatment.
Melatonin treatment also led to a significant increase in calcitonin production on day 1, by canceling the gene expression associated with osteoclastic activity networks. This study showed for the first time that calcitonin in bone is suppressed by microgravity, though serum calcitonin in monkeys in space flights has been previously reported to decrease slightly, and in humans under pseudo-microgravity conditions.
The morphological changes could not be directly measured in space, but were assessed in a microgravity simulation using a 3D clinostat. This showed higher osteoclastic fusion and actin ring formation by day 4, which could be reversed by melatonin.
What do we learn?
These experiments demonstrated a new pathway of melatonin action on bone growth and resorption. Microgravity increases osteoclastic multinucleation and activity, via the increased production of RANKL which induces osteoclastic production and the reduced calcitonin which inhibits osteoclast activity. The total number of osteoclasts does not appear to change, but the RANKL:OPG ratio is increased. Scale osteoblasts are shown to produce calcitonin which is enhanced by melatonin.
Melatonin is able to normalize the levels of both factors by stimulating the secretion of osteoblastic calcitonin during space flight to near-artificial-gravity levels, and could be useful as a preventive therapy against microgravity-induced bone loss in space.
Journal reference:
Melatonin is a potential drug for the prevention of bone loss during space flight. Mika Ikegame, Atsuhiko Hattori, Makoto J. Tabata, Kei‐ichiro Kitamura, Yoshiaki Tabuchi, Yukihiro Furusawa, Yusuke Maruyama, Tatsuki Yamamoto, Toshio Sekiguchi, Risa Matsuoka, Taizo Hanmoto, Takahiro Ikari, Masato Endo, Katsunori Omori, Masaki Nakano, Sayaka Yashima, Sadakazu Ejiri, Toshiki Taya, Hiroshi Nakashima, Nobuaki Shimizu, Masahisa Nakamura, Takashi Kondo, Kazuichi Hayakawa, Ichiro Takasaki, Atsushi Kaminishi, Ryosuke Akatsuka, Yuichi Sasayama, Takumi Nishiuchi, Masayuki Nara, Hachiro Iseki, Vishwajit S. Chowdhury, Shigehito Wada, Kenichi Ijiri, Toshio Takeuchi, Tohru Suzuki, Hironori Ando, Kouhei Matsuda, Masanori Somei, Hiroyuki Mishima, ,Yuko Mikuni‐Takagaki, Hisayuki Funahashi, Akihisa Takahashi, Yoshinari Watanabe, Masahiro Maeda, Hideaki Uchida, Akio Hayashi, Akira Kambegawa, Azusa Seki, Sachiko Yano, Toru Shimazu, Hiromi Suzuki, Jun Hirayama, & Nobuo Suzuki. Journal of Pineal Research. 09 July 2019. https://doi.org/10.1111/jpi.12594. https://onlinelibrary.wiley.com/doi/full/10.1111/jpi.12594