Implantable brain electrodes have been around for quite some time now, both for diagnosing and treating neuropsychiatric conditions like Parkinson’s disease. However, one limitation of conventional probes is their size and rigidity, compared to the soft, gelatinous consistency of the brain.
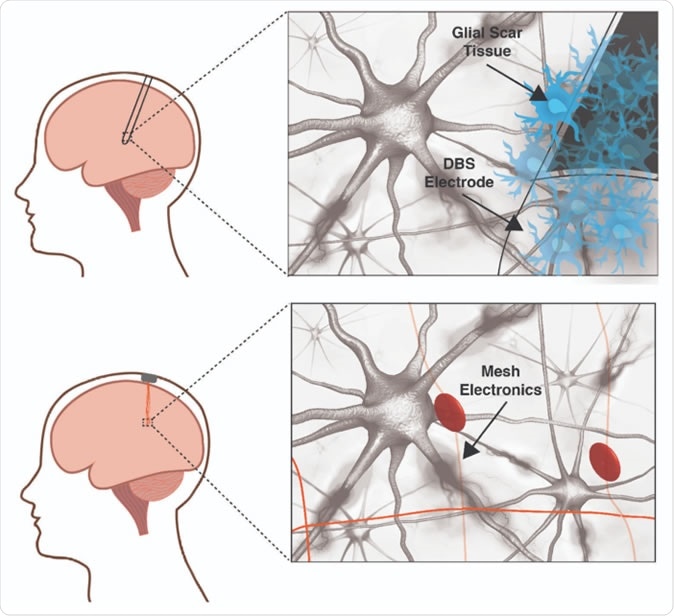
A traditional deep brain stimulation electrode (top panel) provokes an immune response in the brain while a mesh electronic interface (bottom panel) does not. The size and rigidity of the DBS electrode result in chronic inflammation causing glial scarring between brain tissue and electrode, degrading the neural interface. Mesh electronics evade the immune response due to cellular and sub-cellular features and bending stiffness resembling the brain itself. Image courtesy of Shaun Patel and Charles Lieber
Why are implantable electrodes needed?
All neuroscientists agree that the foundation of cognition is the brain, including thinking, emotions, memory, judgment and decision-making. The same applies to all kinds of brain disorders, according to researcher Shaun Patel. Patel became interested in this field after witnessing a 500 ms pulse of electricity conferring an impulsive risk-taker with the momentary ability to make a safer choice, without even knowing how it occurred. The alteration of cognitive thinking, in other words, happened below the level of consciousness.
However, it is difficult for scientists to trace behaviors to specific neurons or brain areas that are dysfunctional, though this could help discover where illnesses such as addiction, Alzheimer’s or Parkinson’s disease originate. Therapy for these conditions is currently limited to drugs and electrical stimulation via implantable electrodes.
Medical treatment is mostly with neuroactive drugs like L-dopa in Parkinson’s disease, which stills the quivering movements that make voluntary actions so difficult. However, the off-target or nonselective inhibition of L-dopa actions causes severe and even unacceptable side effects, on various body systems, including nausea, depression or arrhythmia.
The failure of drug treatment is an indication for electrode implantation to provide Deep Brain Stimulation (DBS). These work by sending automated pulses of electrical energy to paired implants, each about the size of a pencil, which is huge with respect to the brain. These FDA-approved electrodes must be implanted while the patient is awake and able to guide the surgeons during their calibration of the required current intensity that will stop the tremors.
Describing the effect of DBS, researcher Charles M. Lieber marvels, “Almost instantly, you can see the person regain control of their limbs.” However, these electrodes aren’t quite foolproof either, causing inadvertent stimulation of other brain areas at times, which causes disturbing adverse effects such as speech impediments. Just as bad, the brain has an active and vigilant immune system composed of neuroglial cells, which eventually disrupts the electrode’s efficient functioning by smothering it in a protective glial covering. This may also grow so large as to mechanically affect or even kill neighboring neurons. The limited device life caused by unnecessary continuous operation, and the lack of feedback which hampers operational efficiency, are other limitations. While many of these are being individually overcome, the rigid and imprecise neural interface is a basic restriction on further progress.
Implantable rigid electrodes vs mesh electronics
The increased rigidity of even the softest conventional probes causes several problems: chronic inflammation and scarring, mismatched stiffness which leads to a movement of the probe and neuron away from each other relatively quickly, and hinders tracking of the same neuron or circuit; and the solid probe lies uneasily on the quite differently shaped brain surface. This topological difference pushes neurons away from their rightful place, while also preventing repair of broken synaptic connections and blocking the free movement of chemicals in the brain environment.
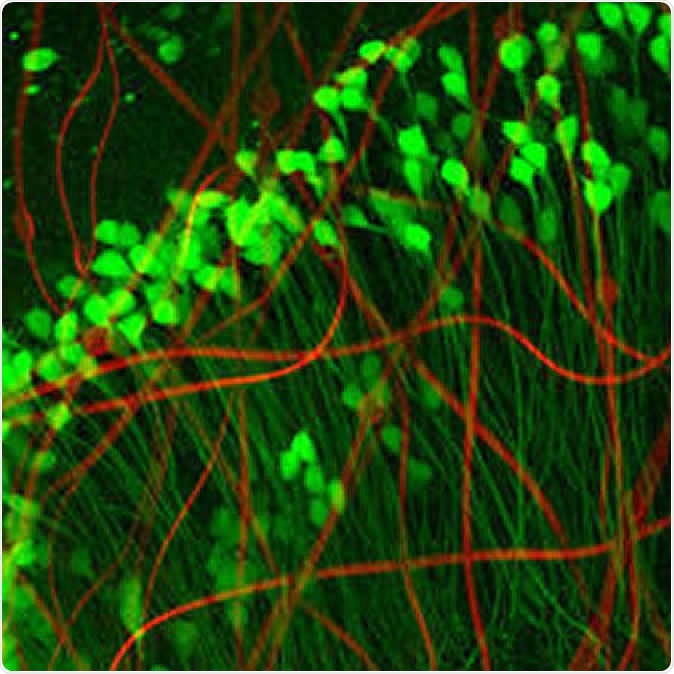
Neuron-like electronics (red) mimic the shape, size, and flexibility of neurons (green), enabling them to maintain symbiosis with native brain tissue (Credit: Xiao Yang, Lieber Lab)
This is where Lieber has made a huge difference with ultra-flexible mesh electronics, which promise to deliver what he calls “precision electronic medicine”. These hardly activate an immune response, but remain very close to the cells they are intended to spy on. As such, they offer a way to obtain reliable information on the communication between individual neurons or its breakdown. This could help elucidate crosstalk between specific subsets of neurons. It is also necessary if any degree of precise regulation is to be achieved via feedback circuits, which requires a two-way transmission of information through the neural device. All this could produce a more accurate and detailed map of the brain circuitry. This could potentially open the door to the successful treatment of any brain disorder.
Moreover, Lieber’s mesh electrodes have a peculiar benefit in their ability to guide developing neurons to the right place, such as injured areas as following a stroke. They could potentially encourage directed neuronal migration to rebuild injured or degenerated areas, and integrate these cells into the right circuits, using differentially modulated successive pulses of electricity.
The risk – and the future
Lieber envisages other mind-blowing advances: special electrodes could be designed to provide precise control over prosthetic or paralyzed limbs, and this could serve as an efficient substitute for damaged brain connections, as well as autocalibration of the signal based on live feedback. He says, “If you could actually interact in a precise and long-term way and also provide feedback information, you could really communicate with the brain in the same way that the brain is communicating within itself.”
Other major would-be players in this field include Neuralink from Elon Musk, which aims at assisting paralyzed patients by providing a mind-computer interface so they can use their computers; Facebook which is looking at texting via word imaging; and Kernel from Brian Johnson, which is focused on increasing cognitive abilities. Lieber emphasizes that his desire is to help people who are sick – and even to prevent the aging-related decline in memory if possible. However, strict ethical control is mandatory lest such mind-altering power be misused. While the benefits are obvious, such as helping people overcome addiction or obsessive-compulsive disorder, the downside is equally plain – the patient’s thoughts are laid open to scrutiny, despite being the innermost stronghold of a person. Secondly, it raises the risk of putting memory, learning ability and other desirable mental traits up on sale, for people who would like to enhance their brain capability.
Meanwhile, scientists like Lieber and Patel continue to refine mesh electronics, by increasing the number of electrodes that can be implanted, designing live feedback systems to enable continuous recalibration, and improving the processing of the voluminous data collected by the electrodes. They urge a blending of multiple disciplines to help advance neurotechnology to the point where it breaches the limit of machine-mind interface. Patel says, “The next frontier is really the merging of human cognition with machines.”
The paper was published in the journal Nature Biotechnology on September 2, 2019.
Journal reference:
Precision electronic medicine in the brain. Shaun R. Patel and Charles M. Lieber. Nature Biotechnology, September 2019, Vol 37, 1007–1012. https://doi.org/10.1038/s41587-019-0234-8. https://www.nature.com/articles/s41587-019-0234-8