A vaccine contains a large molecule, typically a protein antigen, which must be carried across the skin barrier to provoke an immune reaction that will protect the body against the particle that carries the antigen in real life. In the current study, a flu virus protein was targeted. However, the skin is designed to form a barrier to such particles.
Human skin is composed of keratinocytes or skin cells held together by tight junctions, below which there are receptors for a variety of antigens. The tight junctions keep antigens away from these receptors, called pattern recognition receptors (PRR). The scientists therefore looked at the common skin condition called atopic dermatitis or eczema to learn how to make the skin more permeable at one point. Breaking these tight junctions is one way to allow antigens to have successful access to PRRs, thus inducing immune responses to antigens introduced across the skin, or epicutaneous immunization.
In eczema, the skin allows pollens, fungal molds and other particles to cross the barrier, mainly because it contains less claudin-1. This is a protein that helps to keep the barrier strong and reduce skin permeability. Previous experiments by study author Lisa A. Beck showed that reducing claudin-1 content could achieve the goal of increasing the passage of molecules across the skin. This would have to be a transient reduction in permeability so as not to compromise the skin integrity of the patient.
Conventional vaccine shots are undoubtedly effective in inducing a rapid and robust immune response, but the use of needles raises a host of issues. These include the need for trained personnel to administer the shots, the storage of vaccines under safe conditions, safe disposal of needles as biohazards, and the associated pain and fear. All these pose current challenges in broadening the vaccine net in developing countries which are also the areas in greatest need of immunization coverage.
In Beck’s words, “These countries don't have the manpower to vaccinate entire populations. On top of that, there's an aversion to health care in many of these communities. A needle is painful, it's invasive, and that makes things more difficult when you are dealing with a cultural bias against preventative medicine.”
Previous research has focused on electroporation and microneedle-based solutions. However, these require costly equipment, and in the case of the second, may not ensure a uniform, reproducible and reliable application of antigen, besides the possibility that the microneedles may remain in situ and cause inflammation.
The solution
Researchers Miller and Brewer worked out various synthetic peptides to bind to claudin-1, suppressing its activity. Claudin-1 on adjacent cells forms tight junctions by binding through extracellular loops. The researchers produced peptides that could bind to this loop and thus prevent claudin-1 from forming the tight junction at that spot. This would make the skin locally permeable, allowing the antigen to pass through.
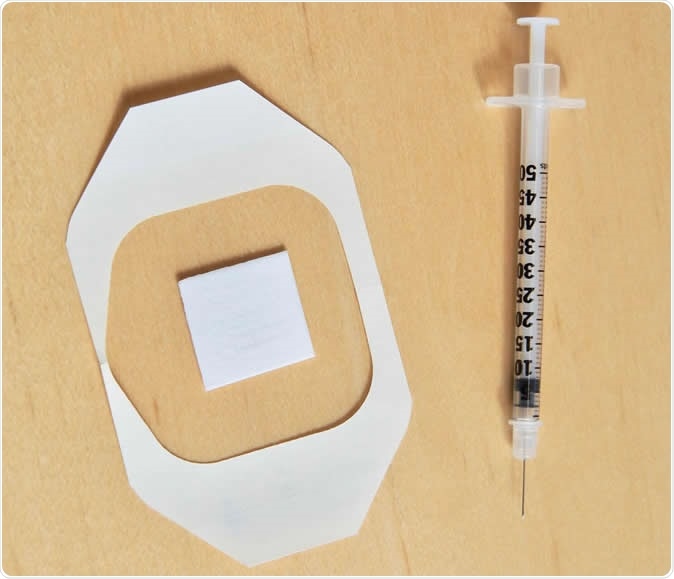
A new needle-free flu vaccine patch revved up the immune system much like a traditional flu shot without any negative side effects, according to a study in the Journal of Investigative Dermatology. Though the research is in the early stages it's an important step toward a technology that could replace needle-based vaccination methods that are difficult to deploy in developing countries. The new patch is pictured below a traditional needle-based flu shot. Image Credit: University of Rochester Medical Center
They then tried out the various preparations on human lung cells, which are known for their strong tight junctions. They found that Peptide 2, as they called it, could successfully disrupt the tight junctions and increase the passage of antibodies.
They then tested it on skin test samples and found they had one which could achieve the needful without causing harm. This was combined with a recombinant flu vaccine on a patch designed to be stuck to the skin. They then set up two mouse experiments, one based on the application of the patch first followed by a traditional intramuscular flu shot. The patch was to initiate an immune reaction and the shot to act as an immunity booster. In the second mouse, the order was reversed, with the patch following the shot.
The vaccine patch in both cases was left on the skin of various mice for intervals ranging from 18 to 36 hours to test for efficacy and toxicity. They measured the rate of water loss through the skin, and found that it was increased in the area of the patch, showing that skin permeability actually increased.
They then tested the strength of the immune response. The use of the patch as the priming antigen seems to be less effective at provoking a strong immune response than the shot, but induced immune memory cells. This was shown by the rapid development of a robust antibody reaction following the booster shot. The reverse protocol also did just as well as the shot at boosting immunity once it was established. At the same time, the researchers observed that the skin started to regain its normal resistance to the passage of organisms beginning from an hour after removal, and was completely normal within a day. It remained healthy over the next three months, with no increased risk of infection.
This suggests that the flu patch may not be ideal in producing a protective level of antibodies in patients who are naïve, that is, have not been immunized or exposed to flu yet. However, such naïve infants can be given a flu shot first, followed by seasonal flu patch application to maintain high levels of immunity against flu. In other words, anyone over the age of six months can use the flu vaccine patch to boost already existing immunity once a flu shot has been taken. This could reduce the need for yearly flu shots.
The future
The safety of the vaccine patch needs to be established using more animal studies to identify the right dose, the duration of application, and any allergic or toxic reactions. Human trials will follow, and hopefully, the researchers say, the vaccine skin patch will replace immunization booster shots in many cases following an initial injection. This will enable rapid large-scale vaccination at a low cost, revolutionizing the practice of immunization.
Another study author, Benjamin L. Miller, says, “Our patch overcomes a lot of the challenges faced by microneedle patches for vaccine delivery, the main method that’s been tested over the years, and our efficacy and lack of toxicity make me excited about the prospect of a product that could have huge implications for global health.”
Source:
Journal reference:
Peptides derived from the tight junction protein, claudin-1, disrupt skin barrier and promote responsiveness to an epicutaneous vaccine. Matthew G. Brewer, Elizabeth A. Anderson, Radha P. Pandya, Anna De Benedetto, Takeshi Yoshida, Thomas A. Hilimire, Luis Martinez-Sobrido, Lisa A. Beck, Benjamin L. Miller. The Journal of Investigative Dermatology. DOI: https://doi.org/10.1016/j.jid.2019.06.145