A research team from the Johns Hopkins Kimmel Cancer Center reports it has discovered a metabolic vulnerability in multiple types of cancer cells that bear a common genetic mutation affecting cellular machines called spliceosomes. In test tube and mouse experiments, the researchers learned that the resulting spliceosome malfunction cripples the cells' chemical process for generating the amino acid serine, making the cancer cells dependent on external (dietary) sources of the amino acid. When mice were fed a serine-restricted diet, their tumors (myeloid sarcomas, the solid tumor version of acute myeloid leukemia) shrank, suggesting that a similar dietary intervention might be helpful for patients bearing the mutation, the researchers say. Among foods high in serine are soybeans, nuts, eggs, lentils, meat and shellfish.
Credit: W. Brian Dalton, M.D., Ph.D.
Previous studies showed that serine deprivation could limit the growth of cancer in lab-grown cells and in mice with tumors, but what cancers would likely respond to this treatment was unknown. Now there is data that cancers with SF3B1 mutations would be good candidates for trying this, says research leader W. Brian Dalton, M.D., Ph.D., assistant professor of oncology at the Johns Hopkins University School of Medicine. "Now we know that SF3B1 mutations are culprits, and that tells us which patients might benefit from a precision oncology diet lacking serine."
A summary of the research was published online in the Sept. 30 issue of The Journal of Clinical Investigation.
SF3B1 mutations occur with relatively high frequency in blood cancers, including at least 30% of patients with myelodysplastic syndrome, 15% with chronic lymphocytic leukemia and 5% with acute myeloid leukemia. It's found at lower levels in solid tumors such as breast, lung and prostate cancers, but the higher incidence of these cancers contributes to an overall incidence of the mutation in about 100,000 patients in the U.S., Dalton notes.
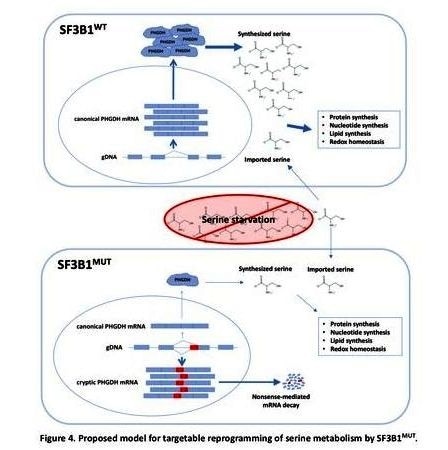
SF3B1 makes a protein that forms part of spliceosomes -- cellular machines in the nuclei that are essential for the correct translation of the genetic code.
Previous studies revealed that mutations in the SF3B1 gene cause the spliceosome to malfunction. Normally, when a gene's DNA is read in the nucleus, a copy is made in the form of messenger RNA (mRNA). These strands of genetic code initially contain segments called introns that need to be removed to create more succinct messages. In normal circumstances, the unprocessed mRNA is exported to the cytoplasm, and it encounters spliceosomes, which splice -- or cut out -- the introns, leaving a clean strand of genetic code to be "translated" into amino acids to form proteins.
Mutated spliceosomes, however, don't follow the splicing cues contained in the mRNA, Dalton says, resulting in garbled RNA messages that are often caught by a proofreading mechanism that destroys the faulty mRNA. The result is a decrease in the protein produced from hundreds of genes.
Dalton and colleagues wanted to know how that decrease affects cellular function. For the new study, they began by genetically introducing the most common SF3B1 mutation into noncancerous human breast cells (MCF-10A) grown in the laboratory. An analysis of the mRNA produced by the cells showed that hundreds of genes were misspliced, and dozens of those had decreased total mRNA expression.
A look at the abundance of more than 5,700 newly produced proteins showed increases, decreases and missplicing.
It showed increased and decreased protein that was the likely result of missplicing of RNA, and in one case, we found a protein with an extra amino acid sequence that was the direct result of missplicing."
W. Brian Dalton, M.D., Ph.D., assistant professor of oncology at the Johns Hopkins University School of Medicine
When the protein changes were assessed overall, the researchers found that many of the protein levels enhanced by the mutation were involved in mRNA processing, while those with diminished levels were often involved in energy metabolism.
Metabolism is simply how nutrients are used to create the molecular building blocks cells need to function and replicate, so it's super important for cancer cells that want to replicate frequently."
W. Brian Dalton, research leader
One metabolic gene with severely affected activity was phosphoglycerate dehydrogenase (PHGDH), an enzyme critical to the creation of the building block serine. Because mammalian cells are equipped with enzymes that can make serine from other molecules, it is usually considered a nonessential amino acid, meaning it does not need to be consumed regularly in an animal's (including a human's) diet. But Dalton wondered if the suppression of PHGDH had changed that.
To test the cells' ability to thrive without serine, the researchers grew mutated cells in a nutritional "soup" in the lab. The "soup" lacked serine and glycine, a precursor to serine. The cells grew more slowly under those conditions and produced little serine and glycine of their own.
When the researchers introduced the SF3B1 mutation into human breast cancer cells (T47D), they found that the PHGDH mRNA was misspliced, and its protein levels had decreased. Furthermore, the tumorlike spheres normally formed by the cells were 30% to 40% smaller when grown in nutrient "media" lacking serine and glycine. Partial growth suppression without serine and glycine was also found when the researchers introduced SF3B1 mutations into multiple types of leukemia cells, and the suppression was relieved when they compensated for the loss of PHGDH either genetically or chemically.
Finally, the researchers tested their observations in mice implanted with two types of human leukemia tumors (HNT-34, MUTZ-3). As expected, the tumors grew more slowly in mice receiving a serine/glycine-free diet.
Most cancer therapies are drugs designed to inhibit a particular protein that has become central to the cancer cells' survival, but the same protein is often needed by healthy cells, so the drugs come with many side effects, says Dalton. "The reliance of these SF3B1 cancers on external serine is a promising trait to exploit because healthy cells maintain their ability to create serine, so they can tolerate its dietary removal," he says.
Dalton, whose clinical expertise is in acute myeloid leukemia and myelodysplastic syndromes (MDS), and his colleague, Amy DeZern, M.D., M.H.S., associate professor of oncology at Johns Hopkins, say they are designing a feasibility study to see if serine levels can safely be lowered in MDS patients with the SF3B1 mutation. They also plan to see if the number of cells with the mutation decreases.
"Early in MDS, disease progression is slow, so there's a good test window for this dietary intervention," says Dalton. "If it works, we would ultimately think about integrating the diet with other standard therapies."
Source:
Journal reference:
Dalton, W.B. et al. (2019) Hotspot SF3B1 mutations induce metabolic reprogramming and vulnerability to serine deprivation. The Journal of Clinical Investigation. doi.org/10.1172/JCI125022.