Among the most deadly killers in the area of cancer, breast cancer is also extremely difficult to treat, once it has spread to the bones. Not only does it metastasize rapidly, but it comprises a variety of cell types and also depends on the input from many other non-breast cells during the initiation, growth and spread of the cancer. This complexity makes it more difficult to develop targeted therapies in this condition.
Now a team of researchers from Salk Institute have produced a revolutionary cell profiling tool and an open database, to help understand the cell-level changes related to normal breast development – and to decipher the divergent development that leads to the formation of a tumor.
These findings were published on October 8, 2019, in the journal Cell Reports. The researchers hope to thereby help lay the foundation of a common web-based data resource that will help to arrive at a more accurate understanding of how to treat breast cancers.
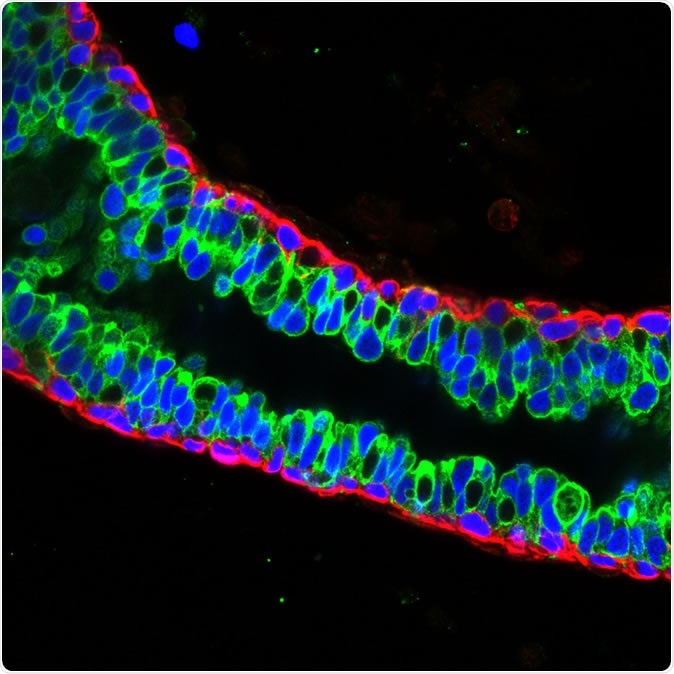
Luminal cells (green) and basal cells (red) in mature mouse breast tissue. Credit: Salk Institute
When the breast has fully developed, it comprises two main types of cells, namely, luminal and basal cells. The former line the mammary ducts and produce human milk. The latter are located around the luminal cells and have both muscle cell-like and epithelium-like characteristics. These are the cells often implicated in breast tumors.
The current study aimed to discover the molecular level changes that determine the differentiation of the immature stem cells into this particular subset of cells. They focused chiefly on the DNA inside the chromatin strands within the nucleus. They examined these strands minutely at different time points, to see how changes in this structure make specific genes more or less accessible to transcription factors, and thus decide which genes are expressed in the form of protein products, driving the development destination of these cells. This is called a molecular map, and helps understand how various different breast tissues come to form during the development and later the maintenance of the adult breast.
How did they do this?
The current study examined breast tissue from both adult mice and mice in the later stages of fetal development, using a technology that allows single cells to be characterized. In this way, the immature cells were tested to determine their features such as basal-like or luminal-like cells. This classification was made on the basis of which chromatin segments were more accessible to transcription enzymes. The scientists found, remarkably, that the destination of each individual cell was already decided before the baby was born. They concluded that this meant that the cells gained the capability of transforming into the predefined cell type just after birth. The directions for this change came through alterations in the level of accessibility of different parts of the chromatin, as well as via crosstalk mediated by cellular molecular signals.
Importance of the study
The importance of this process is clear: if and when it is altered abnormally, tumors may develop. To facilitate this, the team continued to use bioinformatics and machine learning tools to unravel the different developmental processes underlying the formation and differentiation of each of the various features of a normal breast.
They also incorporated the complete set of findings into a free database available online, so that scientists studying cell growth, cell cycles, gene regulation, and other factors that act to determine how stem cells develop into different cell types and attain different stages of development, can find information on which to base new hypotheses on the emergence of cancer cells and the process of tumorigenesis in the breast. This database allows researchers to directly compare patterns of chromatin accessibility and of gene expression during the normal development of the breast with those obtained during other studies. Their aim is to make the findings derived from sound research as widely known and freely available as possible, in order to speed up the development of effective therapies against cancer.
Researcher Geoffrey Wahl says, “In order to understand what goes wrong in breast cancer, we need to first understand how normal development works. This study has provided us with a concrete way of understanding the steps involved in mammary development and reveals a complexity that was not evident by most other methods. This information [will] be a valuable hypothesis-generating resource for the [breast] community.”
Future plans include continuous broadening of the database as more developmental research is completed. This will include information on the development of the breast at more time points, which will, in turn, help find more points at which cancerous changes occur and can be targeted.
Journal reference:
Single-Cell Chromatin Analysis of Mammary Gland Development Reveals Cell-State Transcriptional Regulators and Lineage Relationships Chung, Chi-Yeh et al. Cell Reports, Volume 29, Issue 2, 495 - 510.e6, https://www.cell.com/cell-reports/fulltext/S2211-1247(19)31151-9