Earlier research provides strong indications of the link between gut health and a range of diseases as different as ASD and post-traumatic stress disorder. Many studies have shown that autoimmune diseases are linked to an abnormal gut microbiome, and also to several psychiatric and neurologic conditions. For instance, inflammatory bowel disease, multiple sclerosis and psoriasis are all disorders of autoimmunity, and these individuals have a higher risk of having lower numbers of various gut bacteria, as well as greater odds of anxiety, mood disorders and depression. Common genes also appear to be present in both psychiatric and autoimmune conditions.
Unhealthy gut microbiome and poor learning capacity
In the present study, the scientists looked at the brains of two types of mice with abnormal gut microbiota: mice which had received antibiotics to reduce bacterial growth in the gut, or mice that have been bred in a totally sterile environment to be germ-free (GF). They first exposed these mouse populations to a danger, and then removed it. On studying the learning responses in these mouse populations, they found that both showed a lowered capacity to learn that a danger that threatened them was no longer to be feared (called fear extinction learning). In other words, normal mice develop a reflex fear response to a threatened danger, but over time as the stimulus failed to produce any harm following exposure, their conditioned fear responses decline. This learning was not apparent in the GF or antibiotic-treated mice who continued to show conditioned fear responses over time.
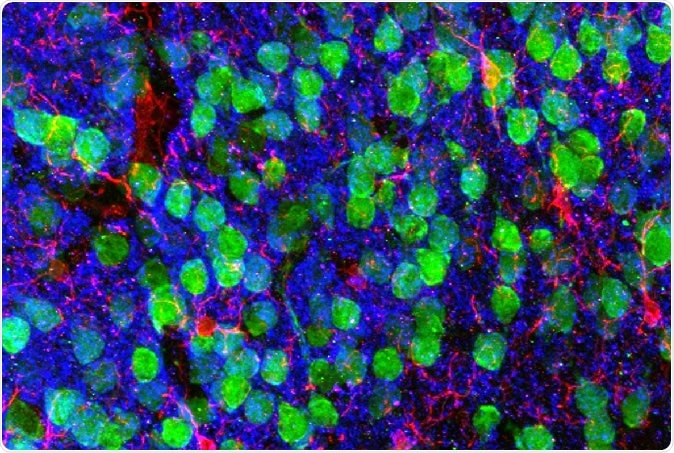
Imaging the brain. Medial prefrontal cortex demonstrating cortical neurons (green), microglia (red), and the post-synaptic marker PSD95 (blue). Image courtesy of Drs. Christopher Parkhurst and David Artis (WCM).
The reason why
To find out why, the researchers sequenced the RNA within microglia, the brain’s immune cells. RNA is the intermediate molecule between the genetic blueprint in the nucleus and the final protein in the cytoplasm, produced from the gene encoded. Thus an RNA sequence shows a gene expressed in that particular cell.
Synaptic pruning
RNA sequencing showed a different pattern of gene expression in the brain cells of these mouse populations, which in turn affected the normal remodeling that occurs as a part of learning. Brain cells form connections or synapses to pass information between themselves. However, as learning proceeds, certain synapses are removed and others added, according to the traffic of impulses along that pathway. This is called synapse pruning and is an important process in learning.
On examining the differences in gene expression in the medial prefrontal cortex of the mouse brain, the scientists discovered that unlike microglia in healthy mice, these microglia did not show normal pruning changes, and this in turn reduced the number of new synapses that were formed during a learning experience. This change negatively affected their capacity to learn.
Postsynaptic dendritic spines were also not remodeled normally, and cue-encoding neurons in this part of the brain failed to show normal activity levels. Along with microglia, excitatory neurons and other brain cell types showed similar changes.
Altered neurochemicals
In addition, the researchers found that there were changes in the levels of four chemicals in the brains of the GF mice. These chemicals are typically associated with neuropsychiatric illnesses such as schizophrenia and autism-spectrum disorder. Changes in these chemicals are therefore intimately linked to alterations in brain functioning, which in turn decides how we sense our surroundings and how we respond to them. The fact that an altered gut microbiome is linked to something as fundamental as brain chemistry is therefore significant in unraveling the underlying pattern. As researcher Frank Schroeder says, “Brain chemistry essentially determines how we feel and respond to our environment, and evidence is building that chemicals derived from gut microbes play a major role.”
Restoration of healthy gut microbiota and the sequel
On to stage 3 – the researchers now tried to restore normal learning capacity in mice by putting back gut organisms to replace those that were lost, in the first group, or by transplanting normal microbiomes, in the GF population. They succeeded if the gut microbiome was restored to normal immediately after birth, with the treated mice showing normal learning patterns. On the other hand, the lack of improvement with later interventions suggests the essential need for signals from a healthy gut microbiome to the developing brain as soon as the baby is born.
Implications
Researcher Conor Liston comments, “This was an interesting finding, given that many psychiatric conditions that are associated with autoimmune disease are associated with problems during early brain development.”
Researcher David Artis says, “The gut-brain axis impacts every single human being every day of their lives. No one yet has understood how IBD and other chronic gastrointestinal conditions influence behavior and mental health. Our study provides a new piece of understanding of how the mechanisms operate.”
The elucidation of the way gut dysbiosis affects the functioning of the brain at molecular and cellular level will, hopefully, accelerate the identification of target molecules or pathways to treat affected humans in the future.
Source:
Journal reference:
The microbiota regulate neuronal function and fear extinction learning. Coco Chu, Mitchell H. Murdock, Deqiang Jing, Tae Hyung Won, Hattie Chung, Adam M. Kressel, Tea Tsaava, Meghan E. Addorisio, Gregory G. Putzel, Lei Zhou, Nicholas J. Bessman, Ruirong Yang, Saya Moriyama, Christopher N. Parkhurst, Anfei Li, Heidi C. Meyer, Fei Teng, Sangeeta S. Chavan, Kevin J. Tracey, Aviv Regev, Frank C. Schroeder, Francis S. Lee, Conor Liston & David Artis. Nature volume 574, pages543–548 (2019). https://www.nature.com/articles/s41586-019-1644-y