Researchers from Israel, in collaboration with others looked at the effects of the malaria plasmodium on red blood cells in vivo in presence of a drug, to understand the workings of the pathogen in its disease causing ability and also lay foundation for development of effective treatment for the deadly disease.
The study results were published in an article titled, “Mode of action of quinoline antimalarial drugs in red blood cells infected by Plasmodium falciparum, revealed in-vivo,” in the latest issue of the journal Proceedings of Nation Academy of Sciences (PNAS).
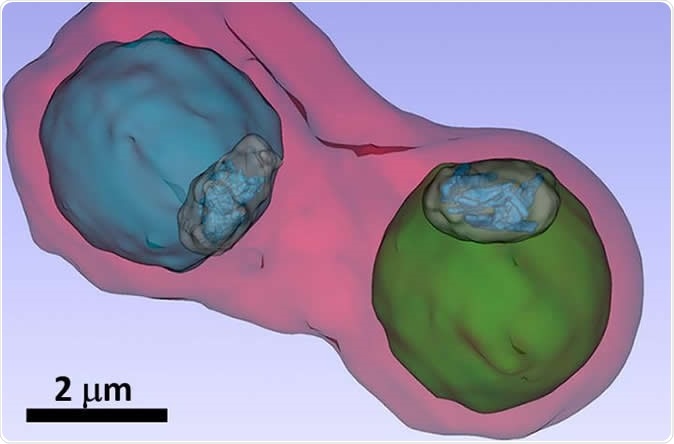
The image shows details such as the vacuole of the parasites (colored in blue and green) inside an infected blood cell. Image Credit: S. Kapishnikov
Organizations have said that 4 in 10 individuals live in regions that are endemic for malaria. Estimates show that nearly 200 million people are infected with malaria annually and the disease kills around 600,000 each year. Malaria is caused by unicellular organisms called plasmodium. These plasmodia are transmitted via the bite of an infected female Anopheles mosquito. The plasmodia are found to settle within the red blood cells of the human host. The parasite metabolizes the haemoglobin within the RBCs and this helps them to grow and multiply until they burst open and infect other RBCs. Artemisinin group of drugs work by preventing the plasmodia multiplying. The researchers looked at the exact workings of the parasite within the RBCs.
Speaking about the significance of the study the team wrote, “The most widely used antimalarial drugs belong to the quinoline family. The question of their mode of action has been open for centuries.” Most speculations on the workings of these drugs include interference of the drugs in the “process of crystallization of heme in the malaria parasite.” However studies have always been done on dried parasites or on model systems and never in vivo on live cells.
The team of researchers explained that within the parasite which has infected the RBCs, there is a digestive vacuole that stores large amounts of haemoglobin. This vacuole releases iron-containing hemozoin molecules. These molecules are toxic for the plasmodia. Thus, in order to survive the plasmodia crystallize the hemozoin molecules. Anti-malarial drugs could target this crystallization process thereby rendering the toxic hemozoin molecules intact and ultimately killing the plasmodium.
Study leader Sergey Kapishnikov from the University of Copenhagen and the Weizmann Institute of Science in Rehovot, Israel, worked in collaboration with Danish, Spanish, French researchers and researchers from Berlin to understand the process of detoxification of the hemozoin by the plasmodium for the first time. They took RBCs and infected them with the plasmodia (Plasmodium falciparum species). These cells with the plasmodia were not exposed to different doses of bromoquine belonging to the quinoline family of drugs.
For their study they had to look at the parasite within the RBCs in vivo rather than in dried forms (wherein the natural processes cannot be studied). In its live form, the in vivo studies can be undertaken using X-ray microscopy at synchrotron sources. This was used by the researchers Stephan Werner and Peter Guttmann and the team at BESSY II. Guttmann said explaining, “The blood samples are flash-frozen for the examination so that we can observe the pathogens in vivo and also produce three-dimensional X-ray tomography images.” Next steps of the study were undertaken at the ALBA synchrotron light source in Barcelona.
To look at the distribution of the elements within the RBCs, the team performed Fluorescence spectromicroscopy at the European Synchrotron Radiation Facility ESRF in Grenoble. Using three-dimensional X-ray images, they could now understand the mechanism of action of bromoquine. Kapishnikov explained, “We see in our images that the bromoquine accumulates at the surface of hemozoin crystals. This should lead to inhibition of the crystal growth and thus disrupt the detoxification process by the plasmodia parasites.”
The authors wrote, “We have provided evidence, albeit indirect, that the drug complexes with free heme, given that the drug binds to the hemozoin surface. This complex accumulates at the membrane of the digestive vacuole, as observed by bromine X-ray fluorescence signal, and possibly spreads to other membranes.” They added, “This model can be generalized to quinoline drugs, such as quinine, which can stereospecifically bind to the {100}, {011}, and {001} faces of hemozoin.”
As a next step of the study the team plans of looking at the mechanism of action of Arteisinin compounds in a similar manner and see if they act in this way by preventing the detoxification of the hemozoin within the RBCs. The team wrote, “The approach adopted in this study can be extended to other families of antimalarial drugs, such as artemisinins, provided appropriate derivatives can be synthesized.”
Journal reference:
Mode of action of quinoline antimalarial drugs in red blood cells infected by Plasmodium falciparum revealed in vivo Sergey Kapishnikov, Trine Staalsø, Yang Yang, Jiwoong Lee, Ana J. Pérez-Berná, Eva Pereiro, Yang Yang, Stephan Werner, Peter Guttmann, Leslie Leiserowitz, Jens Als-Nielsen Proceedings of the National Academy of Sciences Oct 2019, 201910123; DOI: 10.1073/pnas.1910123116, https://www.pnas.org/content/early/2019/10/25/1910123116