Promega Corporation today announced it has entered into a global collaboration with Merck, known as MSD outside the United States and Canada, to develop Promega’s microsatellite instability (MSI) technology as an on-label, solid tumor companion diagnostic (CDx) for use with Merck’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab). The global collaboration will initially seek regulatory approval for the Promega MSI CDx in the United States and China. Plans to seek approvals in additional territories may follow.
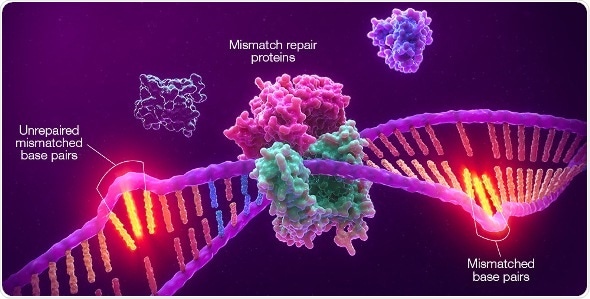
Promega Corporation has entered into a global collaboration with Merck, known as MSD outside the United States and Canada, to develop Promega’s microsatellite instability (MSI) technology as an on-label, solid tumor companion diagnostic (CDx) for use with Merck’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab). Above, an illustration of MSI showing unrepaired mismatches in microsatellite regions resulting from deficiency in the mismatch-repair system (dMMR). (Graphic: Business Wire) Image credit: Promega
“It is gratifying to see our MSI technology have such meaning within the oncology community,” said Bill Linton, president and CEO, Promega Corporation. “Promega developed this technology well over a decade ago and our long-term commitment to R&D helped evolve its use.”
Promega MSI technology has been validated in labs around the world to characterize solid tumor MSI status. MSI testing functionally measures the genomic accumulation of insertion or deletion (INDEL) errors caused by a deficient mismatch-repair system (dMMR) that occurs in certain types of solid tumors, and this screening may be used to better characterize tumors and guide therapeutic choices for MSI-High cancer types. Tumors with MSI-High status have been shown to respond to immune checkpoint inhibitor (ICI) therapies. This outcome may be explained by MSI-driven tumor expression of mutation-associated neoantigens (MANA) that are believed to cause immune cell infiltration into the tumor microenvironment. Tumor induced inhibition of immune cell activity can be overcome with ICI therapies, allowing for tumor cell destruction by the immune cells.
Unlike other DNA-based molecular screening options, Promega MSI technology uses five monomorphic mononucleotides, which is recommended by the National Cancer Institute. Our test uses a sensitive and specific panel of markers for detection of MSI status and offers valuable insight to help inform physicians on how best to treat patients with cancer including those likely to benefit from immune checkpoint inhibitor treatment.”
Jeff Bacher, Ph.D., senior research scientist, Promega Corporation
Promega MSI technology is one of the leading standard tests for MSI status detection in research laboratories and recently achieved innovation status and priority review by the National Medical Products Administration (NMPA) in China. It has been used extensively in clinical research for more than 15 years and is supported by more than 140 peer-reviewed publications. Promega continues to advance the promise of MSI technology globally. In addition to the Merck collaboration announcement, Promega intends to seek regulatory clearance for an MSI in vitro diagnostic (IVD) test in the United States, China and Europe. These products are intended to launch in the first half of 2020 in the United States, China and Europe.