In this Interview, NewsMedical speaks with Amy Landreman about NanoBRET® Technology and its role in cellular target engagement.
Could you start by explaining the significance of understanding cellular target engagement in the drug discovery process and how it impacts the development of therapeutic insights?
Understanding compound target engagement is a crucial step in drug discovery as it provides valuable insights into the interaction between drug candidates and their specific targets.
Accurate measurements of drug-target interactions help researchers optimize drug molecules for increased selectivity and potency, essential for enhancing therapeutic efficacy and reducing potential side effects.
Studying this binding interaction within live cells is key to predicting the clinical efficacy of a drug, as it reflects the physiological conditions that are likely to be encountered in human tissues.
How does NanoBRET® TE technology change the way we understand drug-target interactions in live cells?
NanoBRET® TE technology integrates the precision of biochemical assays with the complexity of cellular contexts, making it possible to generate quantitative and real-time measurements of drug-target interactions in live cells.
The cellular functional and phenotypic assays often used to characterize drug activity report downstream effects of drug-target interactions and can be influenced by non-specific activities, overlapping signaling pathways, and other cellular responses.
In contrast, NanoBRET® TE provides specific compound binding data in the live cell context. This removes many of the unknowns obtained with traditional cellular assays, providing valuable insights that directly support the decision-making process and increase confidence when selecting the most promising candidate compounds for advancement.
What key advantage does NanoBRET® TE offer over traditional biochemical assays in drug discovery?
NanoBRET® TE offers a significant advantage over traditional biochemical assays in drug discovery by enabling the measurement of drug-target interactions directly within the native cellular environment. This is crucial because it allows researchers to observe and quantify how a drug interacts with its target in the presence of cellular components that are absent in simpler biochemical assays.
Traditional assays often fail to account for factors such as the influence of cell membranes, cellular cofactors such as ATP, and protein conformational dynamics that occur within cells.
NanoBRET® TE bridges this gap by providing a real-time, biologically relevant assessment that reflects the true binding activity of a drug under physiological conditions. This approach not only enhances the accuracy of target engagement data but also increases the reliability of predictions regarding a drug's in vivo performance, leading to more effective and safer therapeutic agents.
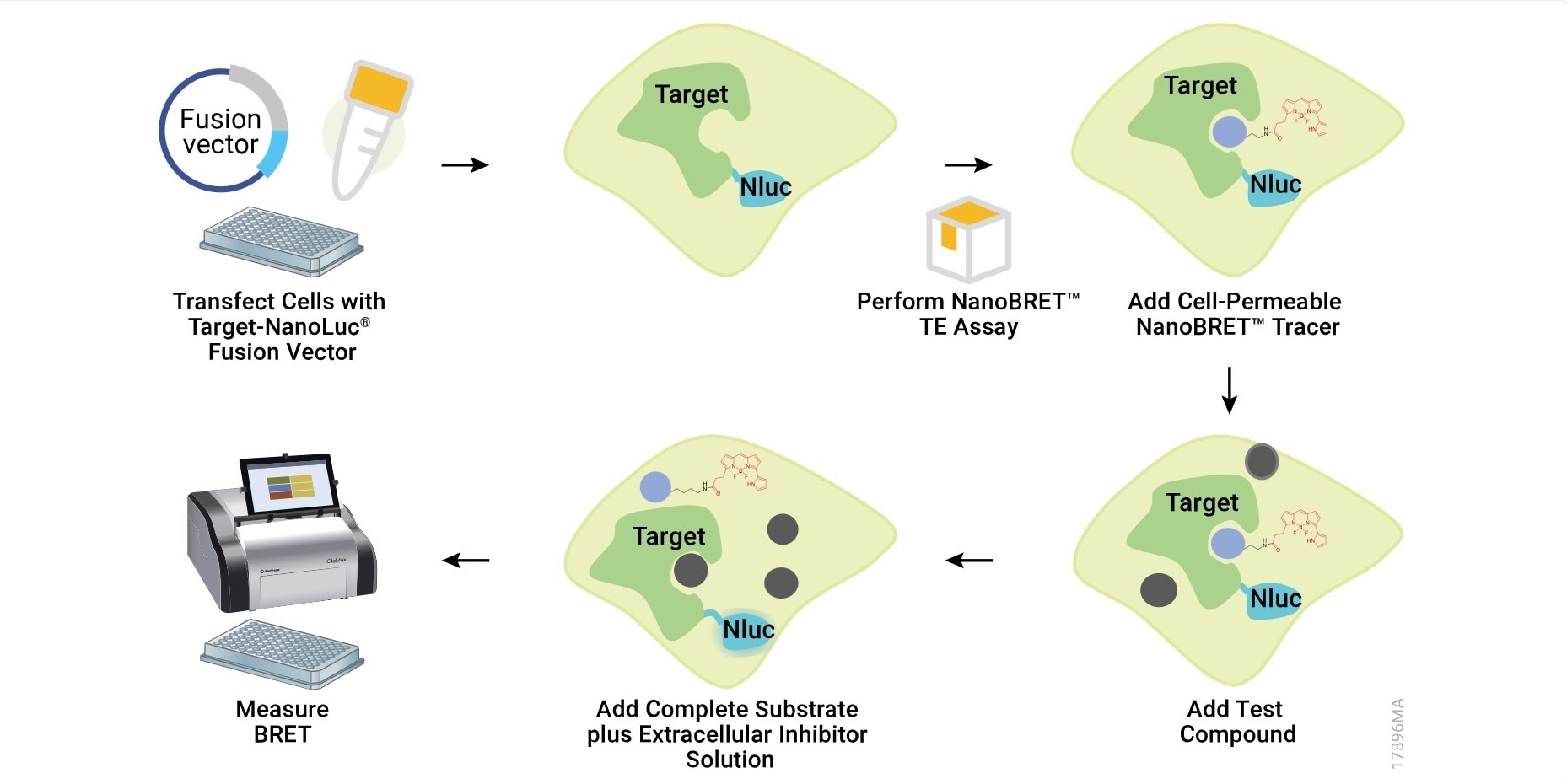
Image Credit: Promega Corporation
Can you explain how NanoBRET® TE quantifies compound-target affinity within a cellular context?
NanoBRET® TE is based on a specialized bioluminescence resonance energy transfer (BRET) technique. This method uses a NanoLuc® luciferase-tagged target protein expressed within the cell and a cell-permeable fluorescent tracer that binds to the target protein.
When the tracer is close to the NanoLuc-tagged protein, energy transfer from the luciferase to the tracer causes the tracer to emit light, which is measured as the BRET signal.
To create quantitative assay conditions, the fluorescent tracer is added to cells at concentrations that are equal to or less than the dissociation constant (Kd) of the tracer. The initial binding of the tracer to the target provides a baseline BRET signal. Researchers then introduce unlabeled drug compounds under study, which can displace the tracer from the target protein, resulting in a reduction in BRET signal.
Under such conditions, the IC50 value measured in the NanoBRET®TE assay approximates the apparent Ki of drug compounds (within 2-fold), providing a quantitative measurement for the compound-target interaction in a live cell environment.
How does NanoBRET® TE measure and impact the selection of clinical candidates?
NanoBRET® TE is often used in the hit-to-lead stage of drug discovery, helping to identify and support the structure-activity relationship (SAR) optimization of promising clinical candidates. The technology provides quantitative affinity results that support the direct comparison of target protein binding across compound collections and enables correlative analysis with effects observed in functional and phenotypic cellular assays.
These rapid, data-driven insights are essential during this iterative stage of drug development, facilitating more informed decisions about which compounds to further optimize or advance.
Additionally, because NanoBRET® TE is based on transfection of the target protein fusion, it can be easily adapted for the study of common disease-driving protein mutations, alleviating the burden of purifying mutant proteins needed for biochemical analysis. This aids in tailoring the SAR process to the development of lead compounds that are more precisely designed for conditions observed in the clinic.
NanoBRET® TE can also be performed in a permeabilized format to assess intracellular compound availability and permeability. This feature is particularly valuable when developing compounds that fall beyond traditional drug design rules, such as proteolysis targeting chimeras (PROTACs).
Information on cellular permeability obtained through NanoBRET® TE can be integrated into the SAR process to enhance compound design for optimal cellular uptake. This approach leads to the development of lead compounds with optimized chemical properties, significantly impacting their potential effectiveness and suitability for clinical use.
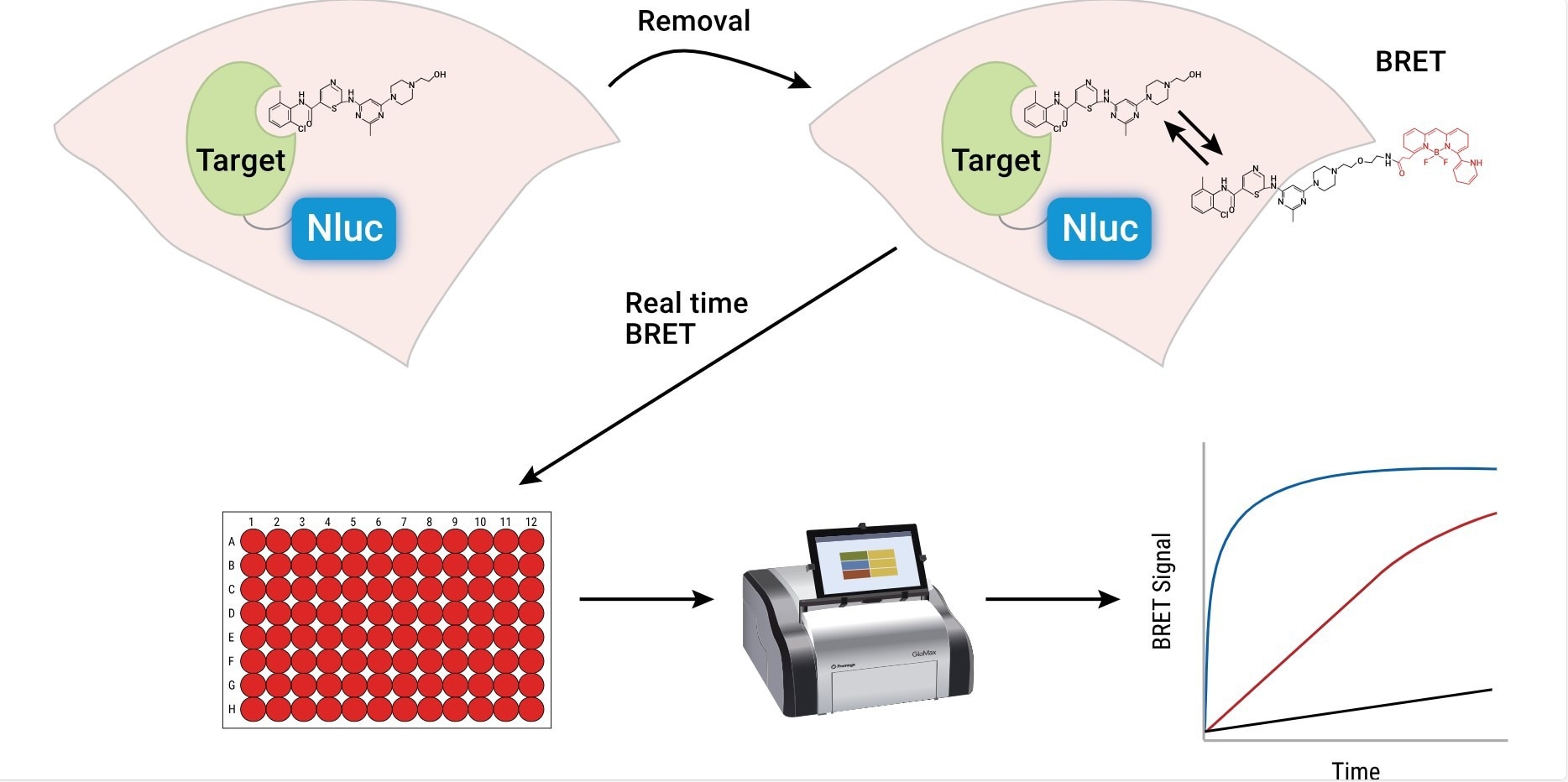
Image Credit: Promega Corporation
In what ways does NanoBRET® TE's capability to assess selectivity advance therapeutic development?
Through the use of appropriate experimental controls, the BRET data can be converted to a measure of the fractional occupancy of the target protein. This enables the quantification of a compound's selectivity across a range of similar targets, such as kinases.
The dual capability to quantitatively assess both affinity and occupancy allows for a more comprehensive evaluation of compound interactions and selectivity within a cellular context so that lead compounds can be optimized for on-target affinity while minimizing off-target effects.
Furthermore, NanoBRET® TE's live-cell format allows for the assessment of compound residence time, providing insights into how long a drug remains bound to its target. This duration can significantly affect a drug's effectiveness and safety profile, influencing the overall therapeutic window.
In addition, as a compound can display differential binding duration among related targets, a drug can be kinetically selective for its target despite having off-target engagement under equilibrium conditions. The ability to measure binding affinity, selectivity, and residence time under physiological conditions ensures that only the most promising, well-characterized candidates advance to the next phases of therapeutic development.
How did NanoBRET® TE unveil trametinib's unique mechanism of action and its therapeutic potential?
NanoBRET® TE played a pivotal role in a study by Khan Z. et al., unveiling the unique mechanism of action and therapeutic potential of trametinib, a MEK inhibitor (MEKi) used in oncology, immunotherapy, and aging-related treatments.
Utilizing structural analysis through X-Ray crystallography, these researchers at the Icahn School of Medicine at Mount Sinai discovered that trametinib distinctively engages with the interface between MEK and the pseudokinase protein KSR (kinase suppressor of RAS). This interaction leads to structural changes in the MEKi binding pocket, potentially influencing how these inhibitors bind and function.
To corroborate these structural findings, the research team employed NanoBRET® TE assays to measure the specific binding of trametinib to the MEK/KSR complex within live cells.
The assay results confirmed trametinib's unique ability to specifically target the MEK/KSR complex, demonstrating greater specificity compared to other MEK inhibitors. Additionally, kinetic analysis via NanoBRET® TE showed that trametinib has a prolonged residence time at the target site, suggesting that its enhanced specificity and extended engagement could reduce side effects and sustain inhibition of MEK activity over longer periods.
These characteristics position trametinib as a more targeted and durable therapeutic option, albeit with ongoing challenges related to drug resistance, which has led to the development of new analogs like Trametiglue to potentially improve efficacy and overcome resistance mechanisms.
What significance does NanoBRET® TE's specificity have in developing treatments for CCNE1-amplified cancers?
Amplification of the CCNE1 locus is prevalent in multiple tumor types, contributing to genome instability and resistance to treatment. By utilizing synthetic lethality approaches, researchers target specific vulnerabilities in cancer cells with amplified CCNE1.
In a significant study by Gallo, D. et al., genome-scale CRISPR-Cas9 screens identified PKMYT1 kinase as a therapeutic target, leading to the development of RP-6306, an effective PKMYT1 inhibitor that demonstrates the potential of precision medicine in targeting specific genetic profiles of cancer.
The NanoBRET® TE assay was crucial in establishing the specificity and efficacy of RP-6306 against PKMYT1, thereby advancing our understanding of cell cycle regulation in cancer contexts.
By comparing RP-6306’s interactions with WEE1, another cell cycle-regulating kinase, the assay confirmed RP-6306's selective inhibition of PKMYT1. This precise measurement of binding affinity within living cells supported the mechanism of action for how RP-6306 induces cell death by prematurely activating CDK1 in CCNE1-overexpressing cells.
As RP-6306 progresses through clinical trials, these findings highlight the role of synthetic lethality in oncology drug discovery, offering new therapeutic options for tumors with CCNE1 amplification and similar genetic conditions.
How does the scalability of NanoBRET® TE support high-throughput drug screening efforts?
In addition to later-stage drug discovery, NanoBRET® TE can be used earlier in the process in high-throughput screening campaigns to identify hit compounds that are candidates for further optimization.
NanoBRET® TE assays are designed to be performed in multi-well plate formats, which can range from small to very large scales, accommodating hundreds or even thousands of samples simultaneously. This capability allows for the rapid and simultaneous assessment of multiple drug candidates under uniform conditions. Researchers can quickly measure and compare the affinity, specificity, and selectivity of various compounds in engaging their cellular targets in a live-cell environment.
The operational efficiency of NanoBRET® TE, which does not require cell lysis or complex sample preparation, facilitates seamless integration into automated workflows. This is particularly advantageous for pharmaceutical companies and research laboratories looking to streamline their drug discovery pipelines.
The ability to conduct these assays in a high-throughput manner not only speeds up the drug discovery process but also helps reduce costs by enabling the early dismissal of less promising candidates. Consequently, NanoBRET® TE's scalability ensures that drug development efforts are both efficient and effective, maximizing the chances of finding successful new therapies.
What are the current limitations of NanoBRET® TE, and how might they be addressed in future research?
NanoBRET® TE, while valuable for drug discovery, faces limitations such as the need for specific tagging of target proteins with NanoLuc® luciferase, which may not always be feasible for all proteins. Future research could focus on developing less intrusive tagging methods that more closely maintain the protein's natural state.
As a step in this direction, the NanoBRET® TE method has already been adapted to the use of complementary NanoLuc® subunits as protein tags to enable the study of target engagement on protein complexes. The use of CRISPR gene editing to incorporate the tag at the endogenous protein loci is another adaptation that would further increase the physiological relevance of this approach.
Further, while NanoBRET® TE is invaluable for assessing drug interactions within cells, its application is primarily confined to in vitro settings. Extending this technology to organoid or in vivo models could significantly broaden its utility, providing insights into how drugs behave in more complex biological environments.
From your Pittcon experiences, how do collaborative discussions shape the future of technologies like NanoBRET® TE?
Collaborative discussions at conferences like Pittcon are instrumental in advancing technologies like NanoBRET® TE. These gatherings bring together a diverse mix of scientists, fostering idea exchanges that drive technological innovation and refinement. Such interactions commonly result in enhanced assay designs, broader application ranges, or tackling challenges like protein tagging and assay sensitivity.
Feedback from end-users during these conferences can also lead to practical improvements in technologies like NanoBRET® TE, making them more user-friendly and tailored to specific research needs. These discussions may reveal emerging applications and new scientific questions, potentially inspiring adaptations such as developing in vivo assay methods for animal studies.
This collaborative dynamic not only guides the technological evolution to meet current and future research needs but also helps identify potential projects and partnerships that could expedite the development and deployment of next-generation technologies.
Finally, reflecting on your experience, how do you envision the future of drug discovery and the role technologies like NanoBRET® TE will play in it?
Reflecting on the evolving landscape of drug discovery, technologies like NanoBRET® TE are poised to play a pivotal role in shaping its future. As drug discovery continues to demand more precise, efficient, and cost-effective methods, technologies that provide real-time, accurate insights into drug-target interactions within the native cellular environment will become indispensable.
Looking forward, I envision that the integration of NanoBRET® TE with other emerging technologies, such as artificial intelligence and machine learning, could further revolutionize drug discovery. These integrations could enhance the predictive accuracy of preclinical models, enabling more targeted therapy options and personalized medicine approaches.
Additionally, advancements in NanoBRET® TE could expand its applications beyond traditional settings to include advanced cell models such as organoids or even in vivo studies, providing a more comprehensive understanding of a drug's performance in complex biological systems.
About Amy Landreman 
Amy Landreman is a Senior Product Marketing Manager in the Life Science Business Unit at Promega Corporation. She earned her BS degree in Botany and her PhD in Molecular and Environmental Toxicology from the University of Wisconsin-Madison. Initially working as an applied toxicologist, she transitioned into the biotechnology industry over 15 years ago. Since then, Amy has held global roles in product technical support, product management, and product marketing. Her work primarily focuses on cell-based assay solutions that facilitate basic research and drug discovery. At Promega, she specializes in the development and strategic commercialization of novel bioluminescence-based technologies that support small molecule drug discovery workflows.
About Promega Corporation
With a portfolio of more than 3,000 products covering the fields of genomics, protein analysis and expression, cellular analysis, drug discovery and genetic identity, Promega is a global leader in providing innovative solutions and technical support to life scientists in academic, industrial and government settings.
Promega products are used by life scientists who are asking fundamental questions about biological processes as well as by scientists who are applying scientific knowledge to diagnose and treat diseases, discover new therapeutics, and use genetics and DNA testing for human identification.
Promega holds significant intellectual property rights and licenses in several key areas that form a foundation for its diverse portfolio including:
- Bioluminescence, including engineered luciferases, luciferase reporter vectors and luciferase substrates
- Short tandem repeat (STR) detection for STR-based cell line authentication, human identification, cell and tissue characterization, and mixed sample detection
- HaloTag® protein labeling and capture technology
Originally, founded in 1978 in Madison, Wisconsin, USA, Promega has branches in 16 countries and more than 50 global distributors serving 100 countries. A cornerstone of Promega business practice is supporting customers, community and employees.