For the first time, researchers from Helmholtz Zentrum München and the University Hospital of LMU Munich show that deep learning algorithms perform similar to human experts when classifying blood samples from patients suffering from acute myeloid leukemia (AML). Their proof of concept study paves the way for an automated, standardized and on-hand sample analysis in the near future. The paper was published in Nature Machine Intelligence.
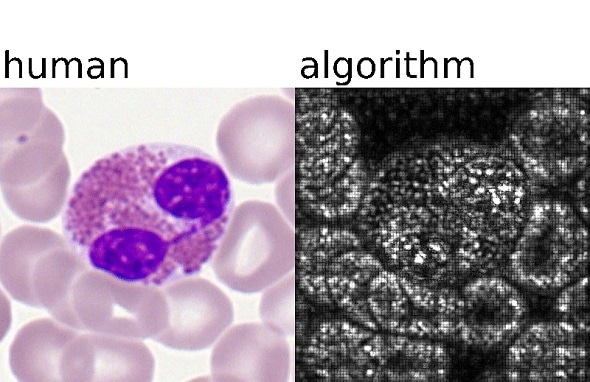
Deep learning algorithms of AI analyses samples in an automated and standardized way. Left: What human experts classify. Right: Pixels important for AI analysis. Credit: Helmholtz Zentrum München / Carsten Marr
Every day, millions of single blood cells are evaluated for disease diagnostics in medical laboratories and clinics. Most of this repetitive task is still done manually by trained cytologists who inspect cells in stained blood smears and classify them into roughly 15 different categories. This process suffers from classification variability and requires the presence and expertise of a trained cytologist.
To improve evaluation efficiency, a team of researchers at Helmholtz Zentrum München and the University Hospital, LMU Munich, trained a deep neuronal network with almost 20.000 single cell images to classify them. The team lead Dr. Carsten Marr and medical doctoral student Dr. Christian Matek from the Institute of Computational Biology at Helmholtz Zentrum München as well as Prof. Dr. med Karsten Spiekermann and Simone Schwarz from the Department of Medicine III, University Hospital, LMU Munich, used images which were extracted from blood smears of 100 patients suffering from the aggressive blood disease AML and 100 controls. The new AI-driven approach was then evaluated by comparing its performance with the accuracy of human experts. The result showed that the AI-driven solution is able to identify diagnostic blast cells at least as good as a trained cytologist expert.
Applied research through AI and Big Data
Deep learning algorithms for image processing require two things: first, an appropriate convolutional neural network architecture with hundreds of thousands of parameters; second, a sufficiently large amount of training data. So far, no large digitized dataset of blood smears has been available, although these samples are used pervasively in clinics. The research group at Helmholtz Zentrum München now provided the first large data set of that type. Currently, Marr and his team are collaborating closely with the Department of Medicine III at the University Hospital of LMU Munich and one of the largest European Leukemia laboratories, the Munich Leukemia Laboratory (MLL), to digitalize hundreds of patient blood smears more.
To bring our approach to clinics, digitization of patients' blood samples has to become routine. Algorithms have to be trained with samples from different sources to cope with the inherent heterogeneity in sample preparation and staining. Together with our partners we could prove that deep learning algorithms show a similar performance as human cytologists. In a next step, we will evaluate how well other disease characteristics, such as genetic mutations or translocations, can be predicted with this new AI-driven method."
Dr. Carsten Marr, Helmholtz Zentrum München
This method showcases the applied power of AI for translational research. It is an extension of the pioneering work of Helmholtz Zentrum München on single cell classification in blood stem cells (Buggenthin et al., Nature Methods, 2017) which has been awarded with the Erwin Schroedinger Prize of the Helmholtz Association in 2018. The study was supported by the SFB 1243 of the German Research Foundation (DFG) and by a PhD scholarship of the German José Carreras Leukaemia Foundation to Dr. Christian Matek.
Source:
Journal reference:
Matek, C., et al. (2019) Human-level recognition of blast cells in acute myeloid leukaemia with convolutional neural networks. Nature Machine Intelligence. doi.org/10.1038/s42256-019-0101-9.