The epithelium of the intestinal wall plays a crucial role in protecting the rest of the body from the billions of microbes that humans are exposed to through food. However, in microgravity, this barrier is disrupted. This not only prevents normal intestinal function but compromises the ability of the intestinal barrier to resist infection even after the astronauts return to earth.

Declan McCole is a professor of biomedical sciences at UC Riverside. (UCR School of Medicine/Carrie Rosema)
Microgravity or near-weightlessness
Microgravity causes the functioning of the body to change dramatically. The changes include genetic, structural and biochemical alterations. These can cause the individual to experience gastroenteritis, and other symptoms of intestinal illness. The body’s immune system also suffers, while bacteria like salmonella become more pathogenic in microgravity conditions, causing intestinal disease.
The reason for this is uncovered in part in the current study, which shows that being in a microgravity environment causes weakening of the intestinal epithelial cells, reducing their ability to withstand the barrier-breaching properties of certain agents. And this weakness was persistently present up to 14 days after the individual returned to a normal environment.
The study
The researchers introduced acetaldehyde, a metabolite produced in the body from alcohol. They chose this agent because alcohol is known to break down the intestinal barrier and enhance the permeability of the gut wall in healthy as well as alcoholic subjects with liver damage.
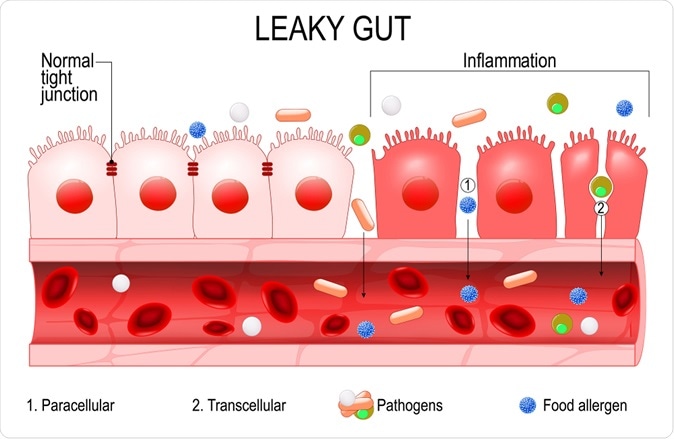
Leaky gut. - Illustration Credit: Designua / Shutterstock
This barrier function is crucial to intestinal health, and its breakdown causes leakiness of the gut. His allows infections and bacterial antigens, or metabolites, to enter the body, causing, as is well known, chronic inflammation. This includes inflammatory bowel disease, celiac disease, type 1 diabetes and liver disease.
The model the team used was a rotating wall vessel, that keeps cells in a setting where they are subjected to constant rotation in a controlled manner. This vessel is a bioreactor, where biological reactions can occur in living cells. The conditions carefully simulate the effect of simulated microgravity on cultured cells taken from the intestinal epithelium.
The findings
The cells were able to grow in a single layer over the microcarrier beads provided. These cells could form tight junctions while the cell polarity, or apex-to-base directionality, was unaffected as well.
After the cells had been cultured for 18 days within the rotating wall vessel, the intestinal epithelial cells were found to show a significant delay in forming tight junctions, the cell-to-cell junctions where individual epithelial cells are joined together to form a watertight barrier that no living organism or biological molecule can cross. Even when they were formed, the pattern was abnormal, probably due to the effect on the passage of tight junction proteins to the cell membrane at the apex of the cell, where the tight junctions are formed. This altered pattern persisted for up to 14 days after removing the cultured cells from the vessel.
When they exposed the cells to acetaldehyde vapor, they saw that barrier defects were observable, with an increase in the permeability of the intestinal epithelial cell barrier. Thus, these cells were more vulnerable to the barrier-disrupting agent than the control cells not exposed to microgravity.
The proteins involved in forming the tight junctions were unaltered in expression, showing that their deficiency was not responsible for the delay. This was seen in cells exposed to acetaldehyde as well as those that were not. However, in acetaldehyde-exposed cells, the tight junction proteins showed disrupted membrane localization under microgravity conditions. Though such a disruption occurred with acetaldehyde in both rotating wall vessel and control cells, it was greater in the first case. Thus, microgravity makes intestinal epithelium more susceptible to functional barrier defects when exposed to a barrier-disrupting agent and causes reorganization of the tight junctions.
The mechanism may probably be explained by earlier studies that show how acetaldehyde impairs the stability of the actin-tight junction protein interaction, causing the tight junction proteins occludin and ZO-1 to be mislocalized.
Conclusion
A microgravity environment can cause healthy cells to develop a barrier defect. Secondly, when such cells are exposed to an agent that is capable of disrupting this barrier, the defect is greatly intensified, and this may upset the whole system of intestinal homeostasis. In fact, this could exaggerate the risk of bacterial infection, chronic inflammation due to exposure to bacterial products, and of rapid serious infection.
Researcher Declan McCole says, “Our study is the first to investigate if functional changes to epithelial cell barrier properties are sustained over time following removal from a simulated microgravity environment. Our work can inform long-term space travel and colonization where exposure to a food-borne pathogen may result in a more severe pathology than on Earth.”
Journal reference:
Alvarez, R., Stork, C.A., Sayoc-Becerra, A. et al. A Simulated Microgravity Environment Causes a Sustained Defect in Epithelial Barrier Function. Sci Rep 9, 17531 (2019) doi:10.1038/s41598-019-53862-3, https://www.nature.com/articles/s41598-019-53862-3