A new study published in the journal Nature Communications shows that it may be possible to reverse gene mutations that cause brain disorders, using a very precisely targeted epigenome editing technique. This type of gene editing does not change the gene’s DNA sequence itself but is focused on correcting epigenome changes only. The condition that was corrected in the study is called the WAGR syndrome and is associated with obesity and intellectual disability in people.
Image Credit: isak55 / Shutterstock
Neuronal genes and brain development
Many disorders of brain development, including autism spectrum disorder, that show up in early infant life or later, are the result of a poorly formed communication pathway between the two brain hemispheres. This in turn is due to chromatin-associated mechanism failures, one of which was corrected in the current study.
The gene they corrected is called C11orf46 and is crucial for controlling the development of the brain. It is responsible for the production of a nuclear protein that regulates certain important proteins that are capable of directing the newly forming long nerve fibers growing out of the developing nerve cells in the right orientation. These direction-sensing proteins help the white matter fibers to bundle together and form the large nerve trunk that traverses the space between the two hemispheres. This is the corpus callosum, and when it is improperly formed, the individual may be disabled, develop autism or another developmental disorder of the brain. Defects in the C11orf46 gene are linked to hypoplasia or poor development of the corpus callosum.
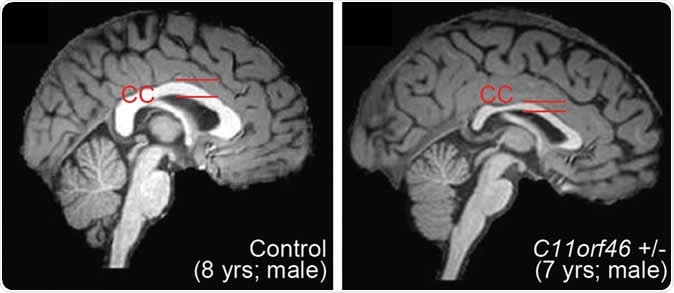
Healthy human brain (left) and brain with WAGR syndrome, in which the corpus callosum is thinner and misformed. Image Credit: Nature Communications
When this gene was knocked down, the projections of axons from one cerebral hemisphere to the other were disrupted. At the same time, multiple genes that code for certain essential events that regulate the development of nerve fibers are overexpressed in these cells. One of these is the Semaphorin 6a gene. This is normalized via epigenetic editing in the present study, producing normalization of the gene expression and restoring normal connectivity in the corpus callosum.
In short, nerve fibers communicating between the hemispheres respond to specific remodelling of the neuronal genetic matter at certain locations.
The WAGR syndrome
Also called the chromosome IIp13 deletion syndrome, the WAGR syndrome occurs when part or all of the gene that forms the C11orf46, in the eponymous chromosome region, is left out accidentally during cell division. The loss of this protein product causes the severe disability that is characteristic of this syndrome.
In the study, a gene-altering tool called short hairpin RNA is used to reduce the production of the protein in the mouse brain. As a result, the nerve fibers in the developing brain could not form themselves into the corpus callosal bundle of white matter, leading to a condition that closely resembles WAGR. This confirms that C11orf46 is important in white matter connectivity, mediating this process via genes that help connect the axons on either side of the corpus callosum.
Mechanism of dysconnectivity in C11orf46 dysfunction
What happens when the C11orf46 gene product is subnormal? The mouse brain in this situation overexpresses another gene that is responsible for the production of Semaphorin 6a, a direction-sensing protein, as well as many other proteins like Doublecortin-like kinase 1 (Dclk1) and SLIT-ROBO Rho GTPase activating protein 3 (Srgap3). In short, this is a regulator of transcription during the developing phase of the brain.
C11orf46 interacts with another region called the KMT-RC. Mutations in C11orf46 prevent this association with this complex and prevent the proper development of the brain. The researchers tested their theory that C11orf46’s binding affinity with this complex could be used to change the pattern of gene expression.
Epigenome editing
One method is by editing the epigenome using CRISPR gene editing, but this might not be effective. Therefore, the researchers adapted this system to alter the regulatory region for the Semaphorin 6a – an epigenomic change.
As a result, they found that the low levels of C11orf46 were now able to bind to the Semaphorin 6a gene region and to downregulate it, reducing its expression. This restored the ability of the nerve fibers to bundle in the same manner as found in normal brains.
Conclusion
This kind of original therapy may help to take forward epigenome editing technology to develop new treatments that can remodel the brain’s neural connections. In fact, this could one day keep developmental disorders of the brain from occurring altogether, according to researcher Atsushi Kamiya. The report ends by saying that such research could “pave the way for novel treatment of neurodevelopmental psychiatric conditions in early life.”
Journal reference:
In vivo epigenetic editing of Sema6a promoter reverses transcallosal dysconnectivity caused by C11orf46/Arl14ep risk gene. Cyril J. Peter, Atsushi Saito, Yuto Hasegawa, Yuya Tanaka, Mohika Nagpal, Gabriel Perez, Emily Alway, Sergio Espeso-Gil, Tariq Fayyad, Chana Ratner, Aslihan Dincer, Achla Gupta, Lakshmi Devi, John G. Pappas, François M. Lalonde, John A. Butman, Joan C. Han, Schahram Akbarian & Atsushi Kamiya. Nature Communications, volume 10, Article number: 4112 (2019). https://doi.org/10.1038/s41467-019-12013-y. https://www.nature.com/articles/s41467-019-12013-y#Sec7