Females are born with a finite supply of eggs and with age the genetic quality of the eggs decline say researchers from Carnegie Institute. The study from researchers Marla Tharp, Safia Malki, and Alex Bortvin was published in the latest issue of Nature Communications. The study was titled, “Maximizing the ovarian reserve in mice by evading LINE-1 genotoxicity.”
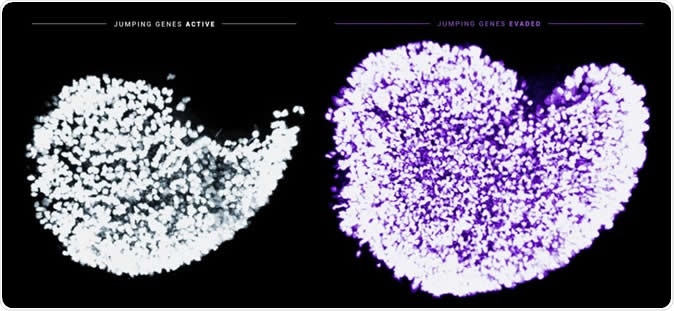
This image displays the dramatic increase in the endowment of immature egg cells in newborn mice when the Fetal Oocyte Attrition is prevented from occurring. Shown in white is an ovary exposed to normal physiological activity of the jumping gene LINE-1. Shown in purple is an ovary that's been treated with AZT to inhibit LINE-1 and mutated to turn off the DNA damage checkpoint Chk2. The nuclei of individual immature egg cells are labeled by a germ cell-specific marker. Image Credit: Marla Tharp and Navid Marvi
The researchers have suggested from the study that the body automatically tries to remove the eggs that have a poor quality in terms of their genetic make-up. Bortvin said in a statement, “Some organisms produce a large number of offspring, many of which don't survive to adulthood; females in these species continually produce new egg cells throughout their reproductive lives. But in mammals, females are born with a fixed supply of eggs and produce few progenies. Thus, each egg is a precious commodity necessitating quality control to ensure wellbeing of her children.”
The experts said that it has been recorded from previous studies that eight in ten eggs of a woman that she was born with, are eliminated during the process of fetal development. This process is called fetal oocyte attrition, or FOA. Bortvin explained that this process of FOA seems to target only the oocytes that are of a genetically poorer quality in order to maintain the genetic health of the offspring and this process has been poorly understood. He added that there is an element called the “jumping gene” that is a transposable or shifting gene called LINE-1. The FOA, he added is associated with this LINE-1.
The team wrote that in most cases the fertility of human females and other mammals is dependent on the “size and quality of the ovarian reserve of primordial follicles, a supply of arrested oocytes and associated somatic cells established by birth.” They added, “Paradoxically, the ovarian reserve of primordial follicles at birth reflects only a smaller share (~20% in humans) of all oocytes initially specified in the fetal ovary. The majority of fetal oocytes generated are lost by fetal oocyte attrition (FOA), a conserved phenomenon among mammals.”
Barbara McClintock from the same institute first discovered the jumping gene. She found that this gene is capable of moving within the genetic work up of the cellular DNA and may even break the genes or introduce new mutations and changes that help the species to survive or live on. This study by McClintock, was followed by Bortvin and colleagues’ research where they noted that this jumping gene could be removed during production of the sperms but were unharmed during oocyte or egg development.
The earlier study by Bortvin and colleagues showed that cells that contained most activity of the gene LINE-1 were removed first and this allowed that immature oocytes which had the lowest risk of dying due to the jumping genes. Malki and Bortvin had also showed before that a particular drug AZT could help inhibit the multiplication of LINE-1. AZT incidentally is a drug used against HIV multiplication as well and is called azidothymidine, an inhibitor of reverse transcriptase. When AZT was used, the deaths of the immature egg’s cells were stopped, the team had found. They concluded that cells with excess LINE-1 activity were thus detected as eliminated by several mechanisms.
In this new study the scientists collaborated and used lab mice which were genetically tweaked to lack a protein called Chk2. This protein plays a role in detecting DNA damage within cells and can work by either repairing the damage or ear marking the DNA damaged cells so that these cells could be programmed for death. These mice lacking the Chk2 proetin were now given AZT to inhibit their LINE-1 function. Results revealed that the number of surviving immature eggs cells rose. Tharp said in a statement, “What's more, the shutting off the fetal egg cell elimination process in this way did not decrease fertility. This provides further evidence that this is a quality control process undertaken to try to maintain the caliber of the available egg supply.”
As a next step the team is working on ways in which these findings could help women suffering from infertility due to premature ovarian failure. They speculate that inhibition of elimination of immature eggs cells could help increase the total egg supply in a female with infertility. The authors also wrote in conclusion, “Future studies analyzing retrotransposition frequency and mutations in a larger pedigree from Chk2−/− + AZT females that did not experience FOA will answer these big questions of whether lack of quality control in fetal oogenesis manifests in the disease susceptibility or adaptive potential of future generations.”
This study was funded by National Institutes of Health.
Journal reference:
Tharp, M.E., Malki, S. & Bortvin, A. Maximizing the ovarian reserve in mice by evading LINE-1 genotoxicity. Nat Commun 11, 330 (2020) doi:10.1038/s41467-019-14055-8, https://www.nature.com/articles/s41467-019-14055-8