These enzymes are called cathepsins and are intended to break down unnecessary protein in the cells. When exposed to certain environments, however, they bring about a higher risk of cancer, atherosclerosis and other conditions. In an effort to stop them from playing this role, they have been blocked using various experimental drugs. The problem is that these drugs are also toxic, with novel and poorly understood side effects.
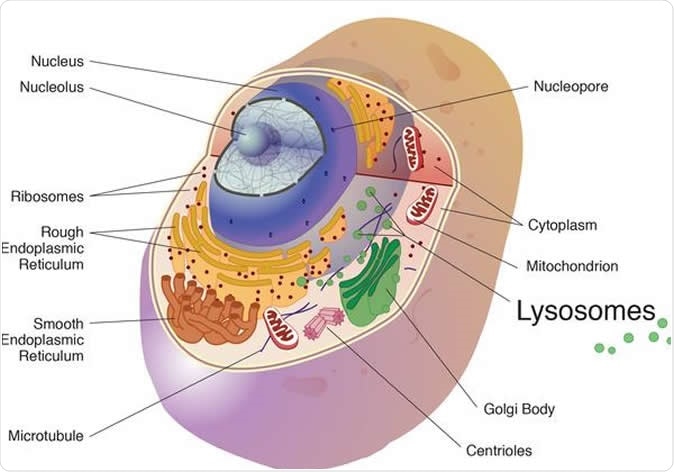
The cathepsins in the study were cysteine cathepsins and are best known for their work in the lysosome, a cell organelle, where they break down unneeded proteins into amino acids. Image Credit: National Institutes of Health
The study
The scientists first attempted to study one cathepsin at a time but could not because of mysterious variations in the outcome. This made them suspect that there could be another reason for this behaviour. Even more, they wondered if this was the same cause for some unexplained drug treatment failures.
In an attempt to better characterize the function of the cathepsins, and the reasons for these unexpected adverse reactions, the researchers chose to look at a model of a biological system that includes three of the enzymes working together, rather than examining them singly.
Using both calculations and experimental observations, they built a computational model that reveals the way a change in one parameter affects everything else.
Cathepsins eat away at collagen and elastin in Manu Platt's Georgia Tech lab. Image Credit: Georgia Tech / Allison Carter
The findings
The study shows that the three cathepsins investigated, namely, K, L and S, are powerful degrading chemicals, breaking down unwanted structural scaffolding material outside the cell. In addition, they also target each other for degradation, offer alternative sites of action rather than the physiological target, and block each other.
The reason why they cannibalize themselves is explained by researcher Manu Platt, “Auto-digestion is my personal favorite. Think about it: You take a group of cathepsin Ks, and they eat each other. Why? Because they're just closer to each other than to what they would otherwise eat.” In other words, this happens because they are designed to break down proteins, including themselves. And this is precisely what happens in a disease condition.
Another important aspect is that the three cathepsins in the study depend on each other to achieve this destructive activity. In normal health the cathepsins used in the current study work within lysosomes, the garbage digesters of the cell, cutting proteins into tiny molecules called amino acids. In certain specialized cells such as the immune cells, cathepsin S helps with antigen recognition, priming the cell to distinguish friend from foe.
However, when these enzymes are present at too high levels, they appear at the wrong sites, going berserk and breaking up even the proteins which build the structure of the body. These include elastin and collagen, the proteins that make up the whole framework of every solid body tissue, from tendons and arteries to the fibrous structure at the core of all solid tissues.
This overactivity is simply an overzealous expression of the normal function of cathepsin K, for instance, which is in charge of degrading bone tissue to retrieve and reuse the calcium. However, in a cancer, the picture changes. As Platt puts it, “When breast cancer comes, those cancerous cells make cathepsin K to destroy collagen around the tumor. And that allows the cells to escape and metastasize to the bone.”
Implications
If the researchers can develop a drug to prevent the tumor-promoting activity of cathepsins, it would be a very beneficial step, since these enzymes play a major role in tendon inflammation, endometriosis, cancer, sickle cell disease and atherosclerosis. Platt continues, “Many cathepsin inhibitor drugs that have failed clinical trials were very finely targeted but caused big side effects, and some of those cathepsin inhibitor drugs did not even cross-react with other cathepsins they were not targeting - which is usually a good thing - so the cause of the side effects was a mystery," Platt said. "By modeling a system of cathepsins, we think we have a good start toward uncovering that mystery."
What he means is that knowing more about how they work in our bodies will be essential to developing an inhibitor to these enzymes where they are unnecessarily active. The researchers also feel they have helped come up with new approaches to this process by their systems model. One example they posit is increasing the activity of cathepsin S specifically where it could break down the other two, namely, cathepsin K and L.
The future
The systems model is available online in the hope that other scientists can also play with these three cathepsins in a group model, varying their levels, the levels of their target enzymes and the amount of inhibitor in the model.
The researchers say they have paved the way for others to carry out further experiments and test the role of inhibitors in different ways. Platt says, “They can set up their own experiments and make predictions, including what inhibitors will do, so they can test inhibitors at varying strengths in this system. They can ask questions that they can't answer yet experimentally then test the model's predictions in the lab.” This is because the systems biology setup allows different inputs to be processed in the form of the final effect on the levels of the cathepsin, and on the amount of degradation. It also shows whether the other cathepsins are active or have been broken down or their activity blocked. The final result will appear in the form of a spreadsheet as well as a report for easy understanding.
Source:
While promoting diseases like cancer, these enzymes also cannibalize each other - https://www.eurekalert.org/pub_releases/2020-01/giot-wpd011720.php