How does the immune system develop its functionality? This has been an ongoing area of research for biologists. Now, a new study published in the journal NPJ Regenerative Medicine in February 2020 shows that in very early fetal life the brain must signal directions to the developing immune system to help it defend the body from infectious agents. This signaling markedly improves the capability of the embryo to survive when faced with bacterial infection.
The researchers used frog embryos for the current study because they are capable of continuing to develop even in the absence of a brain. They then infected these embryos to the bacterium Escherichia coli (E. coli) and observed the outcome. Embryos with a brain were also simultaneously exposed to the infection.
The findings
The experiment showed that in such a situation, the immune system failed to send immune cells to the site of injury or infection. As a result of this poor immune reaction, the embryo’s survival was severely compromised. Only 16% of embryos survived.
When embryos with a brain were observed for their reaction to similar immune challenges, the immune cells were recruited much more effectively to the trauma site or the infection, subduing the bacterial agents. Over half the embryos were protected from the infection.
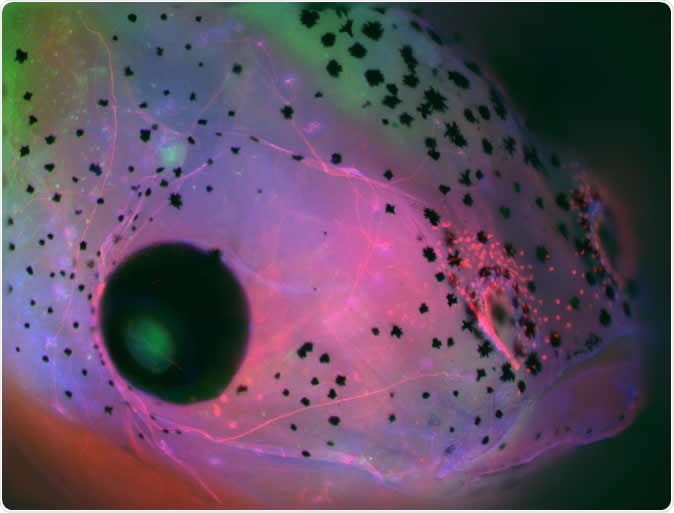
Face of a Xenopus laevis (frog) embryo developed with brain, showing parts of the central nervous system in green (brain on the top down to the middle and retina of the eye to the left) and cranial nerves in red. Image Credit: Celia Herrera-Rincon, Tufts University
The mechanism
The researchers now looked at the pattern of immune cell activation using cell markers or molecule-level identification tags. This showed that the absence of the brain was not the primary culprit leading to the death of brainless embryos, by preventing the development of one or other immune cell type. In fact, the composition of the immune cell population was the same in both groups.
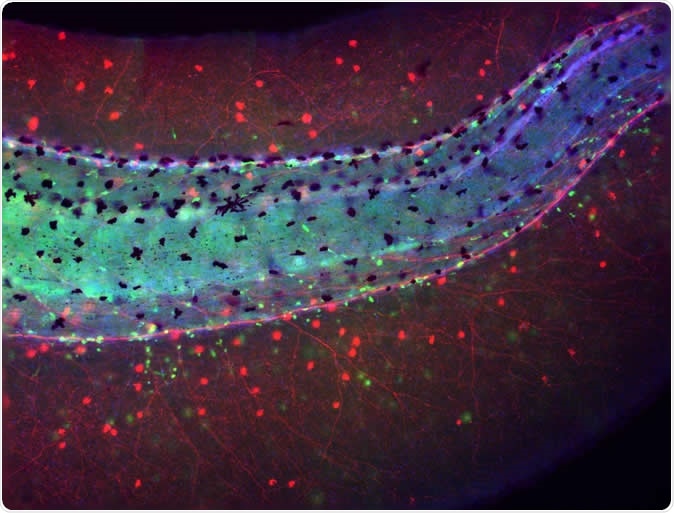
Tail of a Xenopus laevis (frog) embryo developed without brain showing an aberrant distribution of immune cells (macrophages in green) lying in close proximity to the chaotically-sprouted peripheral neural network (nerves in red). Image Credit: Celia Herrera-Rincon, Tufts University
Directing immune cell migration
What really transpired was that the brain’s essential input to the immune system was lacking, so that though immune cells were present, they didn’t ‘know’ they needed to travel to the site of infection.
The fact is that neither brain nor immune system are fully formed in a developing embryo. The immune system at this point comprises primarily an innate component, cells that constitute an immediate response force to invading bacteria without previous training in recognition and attack, nor in response to antibody production. Adaptive immunity, which is induced by prior exposure to antigens, is dependent upon antibody-mediated recognition and attack of invading bacteria.
Nonetheless, innate immunity cells are dependent upon other signals to direct them towards the site of infection or trauma where they are needed. The researchers found that in this situation, the brain supplies the necessary guidance.
Researcher Michael Levin says, “We found that macrophages – innate immune system cells that can swallow up bacteria and destroy them to reduce the burden of an infection – do not migrate appropriately without the presence of the brain. Without the brain and its neurotransmitter signals, gene expression and innate immune system activity go awry, resulting in increased susceptibility to bacterial pathogens.”
Reducing negative impact of infection
Besides this basic role, the brain might also be triggering cell responses such as avoiding the cell’s entry into a programmed death pathway called apoptosis, or reducing inflammatory reactions, both of which modulate the harmful impact of the infection on the cell.
On the other hand, even in embryos that were at a later stage of development, the immune system’s function was further deranged by the absence of brain signals. For instance, when the scientists looked at the immune cell lines that originate in the bone marrow, called myeloid cells, they found that these cells, including neutrophils and macrophages, tended to accumulate in locations unrelated to the site of trauma – such as around purposeless knots of nerve cells and fibers, themselves formed due to the absence of a brain.
Dopamine’s role in immune activation
On closer examination, the brainless embryos had abnormally low levels of dopamine, the neurotransmitter that is key to learning and motivation. Dopamine is also, interestingly, involved in getting immune cells to migrate to the site of infection during the early stages of the immune response. If the minimum number of immune cells is lacking at the infected site, the immune response somehow doesn’t kick off, and the brainless embryos are therefore more vulnerable to the effects of the infection and die.
When the embryo had a brain, the cells actively piled up at the injured site and released cytokines or cell chemicals that helped in healing.
Conclusion
Summing up, researcher Celia Herrera Rincon says, “Our results demonstrate the deep interconnections within the bacteria-brain-body axis: the early brain is able to 'sense' the pathogenic bacteria and to elaborate a response targeted to fight against the cellular and molecular consequences of the infection.”
Journal reference:
Herrera-Rincon, C., Paré, J., Martyniuk, C.J. et al. An in vivo brain–bacteria interface: the developing brain as a key regulator of innate immunity. npj Regen Med 5, 2 (2020). https://doi.org/10.1038/s41536-020-0087-2, https://www.nature.com/articles/s41536-020-0087-2