The flu season is coming, and the frigid temperatures make it worse. The influenza B virus is the causative agent of the common flu, but over the past years, it has become more potent in causing disease. Now, a team of MIT scientists has found and unveiled the structure of the virus, particularly the key influenza protein, in the hopes to develop new drugs to combat influenza.
The Centers for Disease Control and Prevention (CDC) reports that the overall hospitalizations related to influenza this season increased to 29.7 percent per 100,000, which is similar to what happened over the past seasons. Further, a total of 68 children had died due to influenza this season, with 14 deaths occurring during the 2019-2020 season. Overall, pneumonia and influenza mortality have been low while CDC estimates that for this season, there had been 19 million flu illnesses, 180,000 hospitalizations, and 10,000 deaths from flu.
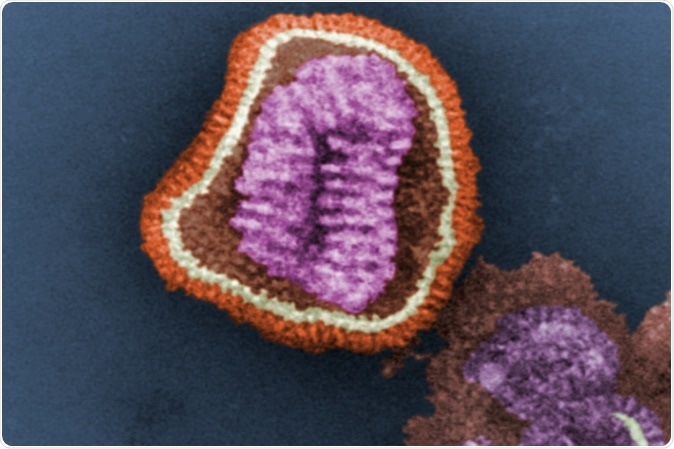
This digitally-colorized transmission electron microscopic image depicts the ultrastructural details of an influenza virus particle. Image: CDC, Frederick Murphy
The protein
Known as BM2, the protein is a proton channel that regulates the acidity in the virus to aid in releasing its genetic material in infected cells.
The researchers believe that blocking the proton channel can help combat infection and block the effects of the virus. Knowing the structure of the protein, particularly its atomic-resolution structure, can help doctors, medicinal chemists, and pharmaceutical scientists to develop compounds and drugs to block its function.
Published in the journal Nature Structural and Molecular Biology, the study sheds light on the structure of the influenza B protein to help in the development of compounds to block its effect on the infected cell.
The three types of influenza virus – influenza A, B, and C, produce a different kind of the M2 protein, which is an ion channel carrying protons through the outer membrane of the virus, known as the lipid envelope. The proteins go inside the virus and will create the internal environment more acidic. If the interior of the virus becomes acidic, it helps the virus release its DNA into the infected cell.
The M2 proteins are very interesting focuses for scientists, in the hopes of finding a cure for flu or treatment modalities to prevent further deaths. There had been many studies about the structure of the M2 protein, but most focused on the type A of the virus.
In the study, however, the team focused on the influenza B M2 protein, which usually dominates the March to April flu season, which accounts for about 67 percent of all flu cases reported by the CDC since September 2019.
Investigating the structure of BM2
The researchers aimed to study what structural differences in the proteins of A and B influenza viruses have. They found that one key difference between the two is that the BM2 channel allows the protons to flow in either direction, while the AM2 only allows the protons to flow into the envelope of the virus.
To land to their findings, the researchers studied BM2’s structure by embedding it into a lipid bilayer, which is akin to a cell membrane. They used nuclear magnetic resonance (NMR) spectroscopy to investigate the structure with atomic-scale resolution.
They discovered that the M2 channel is made of four helices, wherein the alignment chances depending on how acidic or alkaline the environment outside the viral envelope is. If the pH is high, the helices begin to tilt by approximately 14 degrees, and if it decreases, the tilt increases to about 20 degrees. With the motion of the helices, mimicking a pair of scissors, it allows water to enter the channel.
They found that the BM2, unlike the AM2, has an extra histidine at the virion-facing end of the channel. The scientists believe that this explains why the protons can flow in both directions through the channel.
“These results indicate that asymmetric proton conduction requires a backbone hinge motion, whereas bidirectional conduction is achieved by a symmetric scissor motion. The proton-selective histidine and gating tryptophan in the open BM2 reorient on the microsecond timescale, similar to AM2, indicating that side-chain dynamics are the essential driver of proton shuttling,” the researchers concluded.
Funded by the National Institutes of Health, the study has revealed the structure of BM2 in its open and closed state, paving the way for finding a compound to finally block it.
Source:
Journal reference:
Mandala, V.S., Loftis, A.R., Shcherbakov, A.A. et al. Atomic structures of closed and open influenza B M2 proton channel reveal the conduction mechanism. Nat Struct Mol Biol (2020). https://doi.org/10.1038/s41594-019-0371-2