CAR-T therapy involves genetically engineering patient T-cells so that they express a chimeric antigen receptor (CAR).
CARs consist of a protein that binds to cancer cells, usually an antibody, fused to the signaling domain from a T-cell receptor (TCR). The idea is that a killer T-cell expressing the CAR engages cancer cells and eliminates them.
Why is this treatment limited in use and success rates?
CAR-T therapy has worked well as a salvage treatment for chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia.
Professor Carl June’s group presented results using a CAR that saw the B-cell marker CD19 across two seminal papers published in the prestigious New England Journal of Medicine.
CAR-T therapy has not worked for solid tumors, which is what makes up most cancers. It has not worked for myeloid leukemias as there are no recognizable target molecules that can be targeted with CARs on these cancers.
What is MR1 and how does it differ from HLA?
MR1 is a nonclassical major histocompatibility complex (MHC) molecule. Classical MHC molecules are called HLA in human. HLA molecules present small parts of proteins (called a peptide) to T-cells.
The structure of MR1 suggested that it might also present intercellular antigens to T-cells. Indeed, it is already known that MR1 can present intermediates in vitamin biosynthesis to T-cells called MAITs.
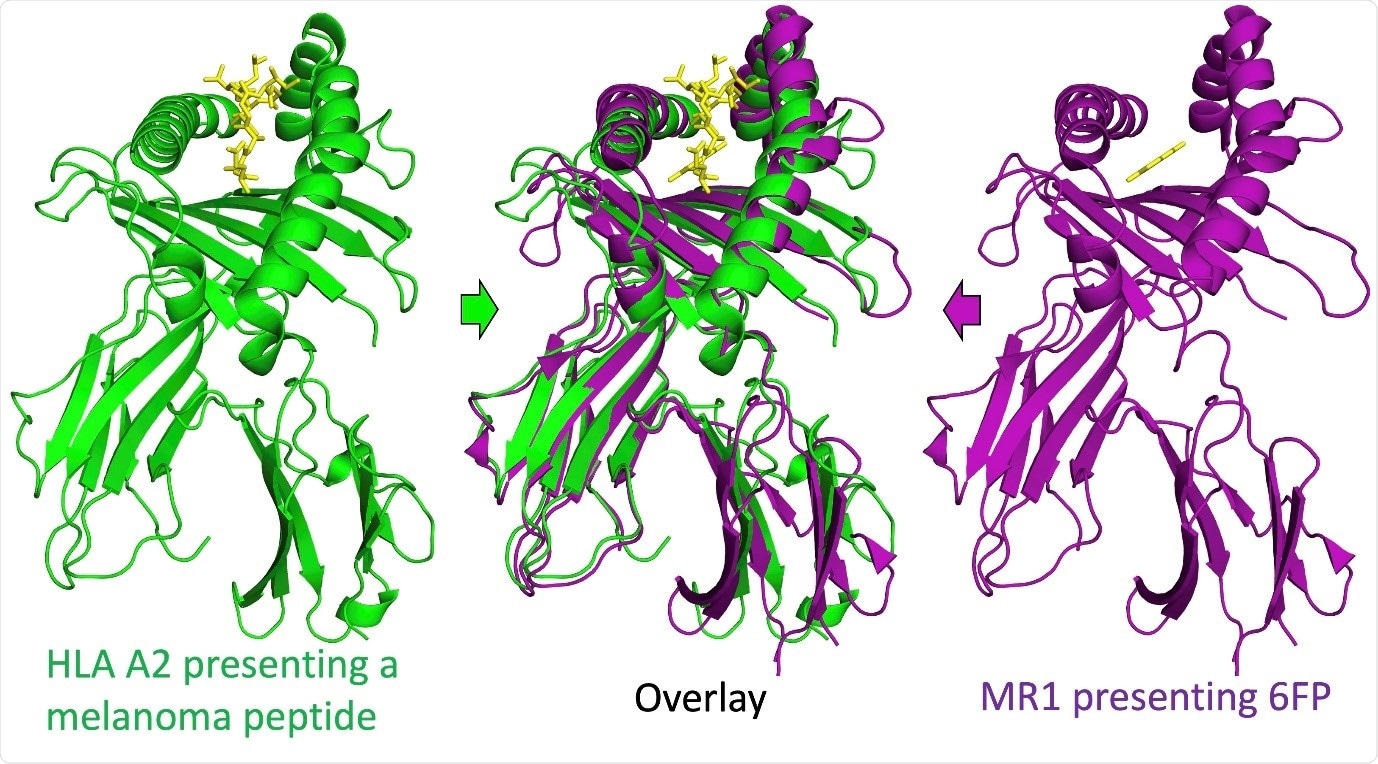
Image Credit: Aaron Wall, Cardiff University
Please describe your recent research where you used MR1 as a target for T cell receptors.
We fortuitously identified a T-cell that could kill most cancer types without the need for a specific HLA.
It was obvious that the mechanism by which this T-cell was recognizing cancer cells was new and worthy of further exploration. To identify what this T-cell was recognizing on cancer cells we used whole-genome CRISPR to knockout each gene in the cancer cells individually before turning our T-cell on these targets.
The T-cell killed all the cancer cells except for a few. We reasoned that some of these remaining cells might be knocked out in the molecule that the T-cell was using to identify the cancer cells. This approach identified MR1 as the target molecule.
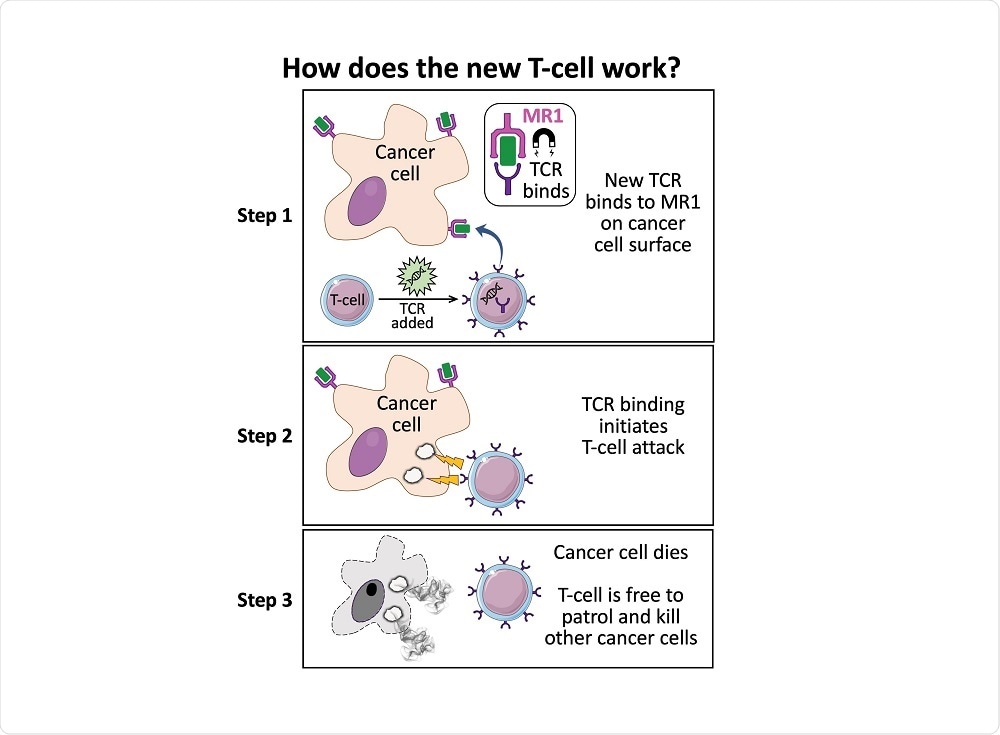
How does the TCR you have discovered, have the ability to distinguish between normal cells and cancerous cells?
We do not yet know the precise molecular mechanism by which this T-cell distinguishes a cancerous cell from a healthy cell.
Our experiments indicate that MR1 is binding to something from the cancer cells that are recognized. If we use a molecule that we know binds to MR1, it can compete with the component being presented from the cancer cells, and block recognition.
Are there any cancer types that do not express MR1 and would, therefore, be unsuitable for this treatment?
All body cells are thought to have the capacity to express MR1. However, our new T-cell did not recognize all types of cancer so there are limits.
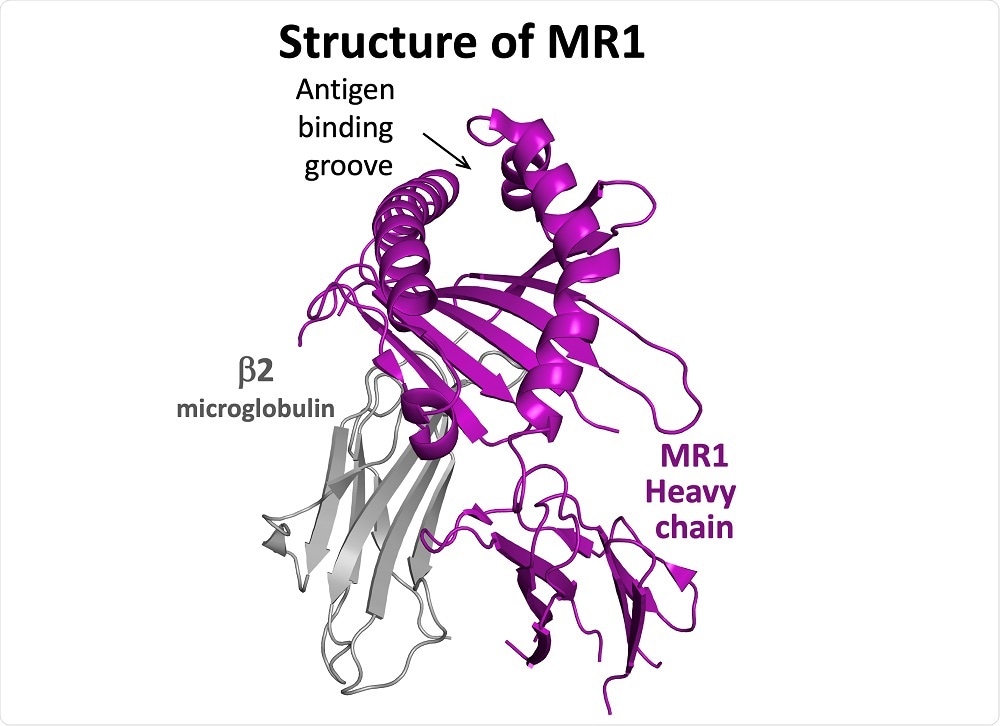 Image Credit: Aaron Wall, Cardiff University
Image Credit: Aaron Wall, Cardiff University
What are the benefits of having a ‘one-size-fits-all’ cancer therapy?
Many benefits can be envisaged. Firstly, it means that the same therapy could potentially be delivered to many patients thereby reducing the cost.
It also provides the possibility of a simplified pathway through the regulatory framework; once a therapy has been shown to be safe and effective for one cancer it can be quickly tested and rolled out for other cancers.
Are there any limitations that need to be overcome before this can be moved into clinical trials?
The most important thing is to show that this T-cell does not recognize any healthy cells. So far, we have tested it with over 20 healthy cell types but there are over 200 different healthy cell types in the human body. Attack on just one of these cell types could result in severe “off-target” toxicity.
Other factors might impact whether the approach will be effective as the T-cells will need to be able to traffic to cancer, penetrate cancer and overcome the immunosuppressive microenvironment that solid cancers can establish.
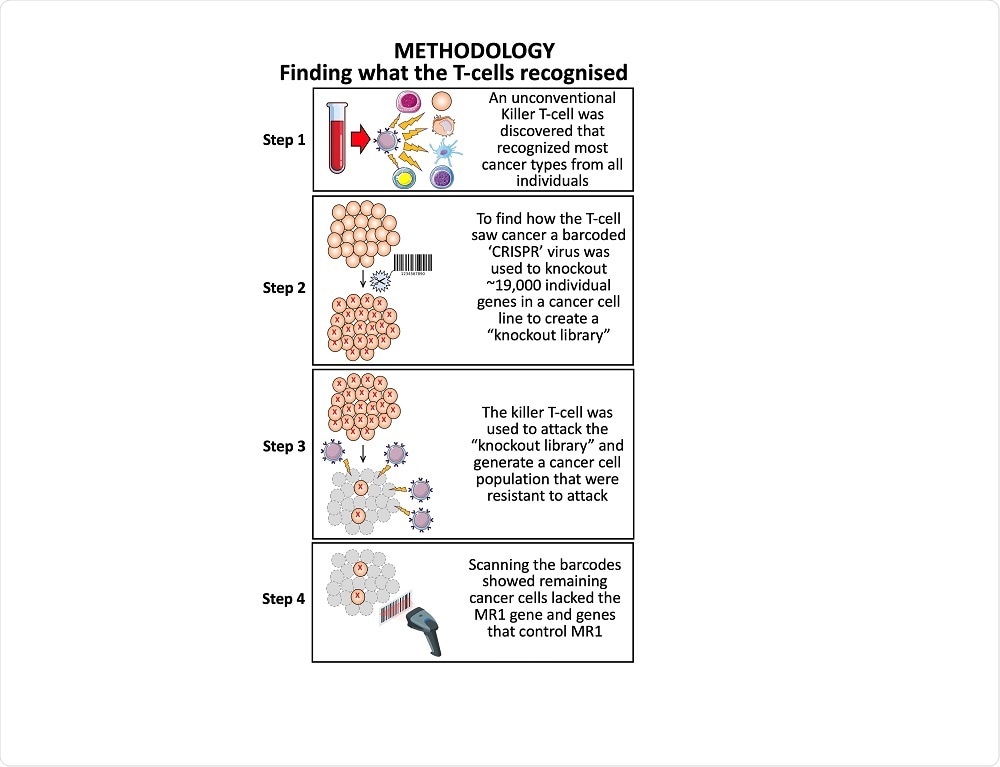
What made you want to focus your research on T-cells?
I focus on the T-cell receptor (TCR) because it sits at the heart of most human pathology. The TCR orchestrates immunity to infection. We would not last a week if we did not have T-cells.
The importance of T-cells can be seen when HIV lowers the number of just one type of T-cell and causes AIDS. T-cells are also essential for effective vaccination and, we now know that they can protect us from cancer.
T-cells also have some very important negative effects as they are responsible for the acute rejection of transplanted organs. I also think they are the root cause of all autoimmune diseases. These, often debilitating diseases, never get better and are responsible for a sizeable fraction of healthcare spending in developed countries.
I believe that dementia and similar diseases are autoimmune diseases and that T-cells could play an important role in initiating such diseases. It is also becoming apparent that T-cells play a key role in many allergic reactions and that when they respond to molecules that they should not recognize they can cause diseases such as celiac disease.
Where can readers find more information about your research?
About Andrew Sewell, Ph.D.
Andrew Sewell’s research career began at the University of Liverpool. Here, he applied his training in chemistry towards phytoremediation strategies, whilst watching football in his spare time.
Andy then relocated to the University of Utah working on gene activation by environmental stress. Here, he also worked on improving his skiing skills.
In 1995, he moved to Oxford to further his research. He began working on strategies that HIV and other viruses employ to subvert human T-cell immunity.
Then, in 2006, Andy moved to Cardiff and took up the position of a Distinguished Research Professor in Cardiff’s School of Medicine. He has since stayed in Cardiff and is currently a Welcome Trust Senior Investigator.
His current research now focuses on T-cell antigens and receptors. This has allowed his research to cover many different research areas including; transplant tolerance, autoimmune disease, cancer immunotherapy and immunity to infection.