The COVID-19 (coronavirus disease) outbreak has taken nearly 1,900 people and infected more than 73,000 people, mainly in China. It has spread to 28 countries, prompting scientists to develop potential therapies to treat deadly viral infections. Now, Chinese doctors are using plasma therapy to treat coronavirus patients.
The promising approach is valid, according to the World Health Organization. In plasma therapy, the doctors infuse the plasma from a recovered patient to a currently infected patient in the hopes of boosting the immune system’s fight against the pathogen.
Doctors in Shanghai are using plasma therapy to help critically ill patients to recover. In the Shanghai Public Health Clinical Center, there is a specialized clinic intended for the administration of plasma therapy.
Professor Lu Hongzhou urge recovered patients to donate blood after they pass screenings for other diseases such as hepatitis B or C or HIV.
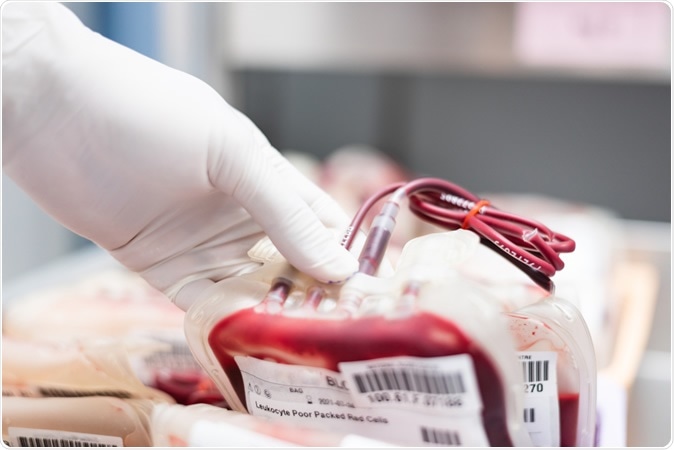
Blood plasma. Image Credit: Komsan Loonprom / Shutterstock
Generate antibodies against the infection
Convalescent plasma has been proven effective and lifesaving in the fight against infectious diseases, such as rabies and diphtheria. Dr. Mike Ryan, the World Health Organization (WHO)’s head of health emergency program said that convalescent plasma is a valid approach against the coronavirus.
The hyperimmune globulin concentrates the antibodies in a patient who recovered from the infection, providing a new victim’s immune system a boost of antibodies. This way, they have the power to survive the infection, especially in a challenging phase.
“So, it must be given at the right time, because it mops up the virus in the system, and it just gives the new patient’s immune system a vital push at the time it needs it. But it has to be carefully timed, and it’s not always successful,” Dr. Ryan explained.
“So, it is a very important area of discovery, and I believe they are starting trials on that in China. But it is a very valid way to explore therapeutics, especially when we don’t have vaccines and we don’t have specific antivirals,” he added.
Plasma therapy has been used in the previous Ebola virus disease outbreak in West Africa in 2014 to 2016. The therapy has shown promise in providing care for patients in the early stages of the disease.
Though there is no proven treatment for these diseases, such as the coronavirus disease and the ebola virus disease, whole blood collected from patients in the convalescent phase of infection has been used as a treatment. For EVD patients, the therapy showed promising results in a small group of EVD cases.
Calling for survivors
China’s state-owned medical products maker, China National Biotec Group Co., has been collecting plasma from the blood of those who have recovered from the coronavirus. The plasma, which contains highly potent antibodies, has been used to treat more than ten critically ill patients since Feb. 8. The company claimed that the patients showed improvements within 24 hours, manifesting reduced inflammation and viral loads, with better blood oxygen levels.
Doctors in Shanghai encourage all survivors of the coronavirus disease to donate plasma for the treatment of other infected patients in the coming weeks. In Shanghai alone, about 124 patients have recovered from COVID-19, while 14 showed a willingness to donate for other coronavirus patients.
Meanwhile, since the beginning of the outbreak, a total of 217 medical teams comprising of more than 25,000 health workers were sent to Hubei Province, the epicenter of the deadly epidemic. Two large hospitals have been built in just ten days, while China built nine makeshift hospitals for patients with mild symptoms in Wuhan City, admitting more than 5,600 patients.
Experts say that the outbreak of new cases toll has decreased compared to the numbers in the previous weeks while the number in other provinces, such as Huanggang and Xiaogan, has remained relatively high, and it’s increasing rapidly.