It is commonly observed that the brain in patients with Alzheimer's disease (AD) often contains fibrils of tau protein, which build up within the brain neurons to interrupt proper communication between them. However, it is far from clear what these deposits mean and how they form.
Tau is unique among proteins in that it lacks a definite structure. Described as being "intrinsically disordered," it is capable of adopting several shapes. Researcher Malte Drescher explains, "We can imagine it like a rope: it can be sometimes elongated, sometimes bent, sometimes looped." Yet tau likes to keep a structure that shows a typical recurving, rather like a conventional paperclip.
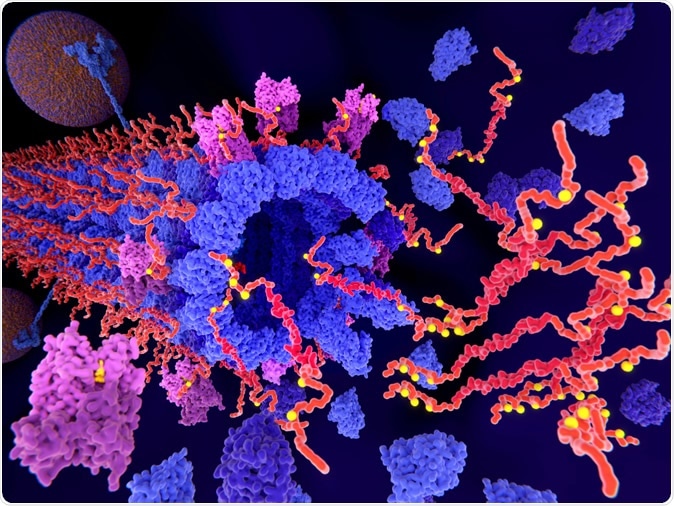
Pathological phosphorylation (yellow) of Tau proteins (red-orange) by kinases (violet) leads to disintegration of microtubuli in an axon and to aggregation of the tau proteins. Image Credit: Juan Gaertner
Molecular chaperone
The tau protein is only about a billionth part of a meter and is therefore far smaller than can be visualized by a light microscope. Its intrinsic disorder makes it very difficult to elucidate its structure using otherwise routine and extremely useful studies such as X-ray analysis of molecular structure, due to the great flexibility such an attribute bestows.
The tau protein undergoes a structural change the moment it meets a molecule called Heat Shock Protein 90. The HSP-90 is a molecular chaperone. Typically, such molecules are necessary to bring nascent proteins into their prescribed structural form.
However, the scientists began to wonder how such molecular chaperones function and what they do, with proteins such as the tau protein, which is intrinsically disordered and therefore has no 'right' structural form.
This motivated the current study, where the researchers explored the unusual role played by HSP-90 in achieving the final form of the tau fibril.
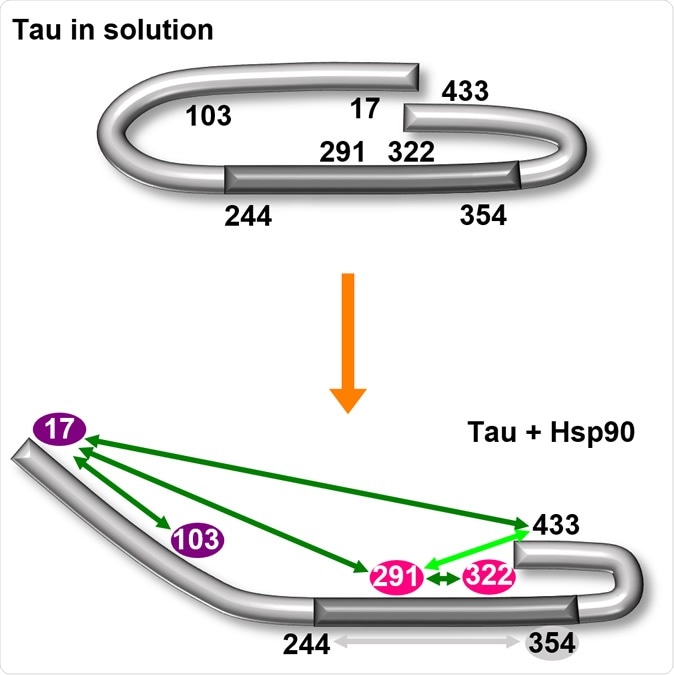
The chaperone HSP-90 opens the characteristic paper clip conformation (top) of the tau protein. The central region of the molecule is thereby exposed (bottom). Illustration: University of Konstanz
The findings
The researchers discovered, using structural analysis tools that the HSP-90 induces a particular conformational change in the tau protein. The result is the opening up of the paperclip-like structure, with the parts representing the clip's brackets bent outwards. This single change allows the interior of the middle part of the paperclip structure to become accessible to other agents.
How is this important? Researcher Sabrina Weickert explains, "This area is known to be responsible for aggregation, i.e., for attachment of further tau proteins to the molecule." The laying bare of this area allows stackable attachment of multiple tau molecules to each other in a beautiful matching arrangement, called oligomerization.
The fact that HSP-90 induces oligomerization was unexpected because molecular chaperones are mostly responsible for doing the opposite. Drescher describes the role of a chaperone in this way, "It is supposed to bring a protein into a defined form and under no circumstances contribute to the formation of a 'protein pile.'
Friend or foe?
The next question the scientists asked is whether the presence of the HSP-90 triggers the formation of the tau fibrils. This would be a strange situation, indeed, where the chaperone itself leads to the development of AD, rather than the exact reverse. While this is being reserved for a future study, the team expects that they will find the opposite is true. Drescher explains, "I would argue exactly the other way round: It could even be a trick the body does to prevent Alzheimer's."
The basis of his belief is the curious fact that the oligomers of tau formed in association with HSP-90 are characterized by one crucial aspect. In essence, they do not continue to oligomerize to form the typical Alzheimer fibrils. As a result, argues Drescher, "The oligomerization by HSP-90 might be a defense mechanism in which the chaperone forces the tau proteins into the form of small oligomer layers."
While such oligomers are not beneficial to the neuron, they do, at least, forestall the formation of the more prolonged and more unmanageable tau fibrils that are typical of AD. This function is in keeping with the forecasted function of a molecular chaperone. It keeps tau from forming the unwanted and toxic Alzheimer's fibrils by acting pre-emptively to usher the protein into a relatively more benign stack of oligomerized tau.
The study
To bypass the difficulties of structural determination using X-ray analysis, the investigators exploited the power of spin labeling. This very advanced technique, called electron paramagnetic resonance, is built around the use of tiny molecules called spin labels, which attach to essential positions of the tau protein, such as the outer brackets of the paperclip arrangement. These minute molecules are functioning as probes, reporting back on the distance between themselves due to their magnetic nature.
By measuring how far apart the probes are at any time, based on their magnetic interaction, the scientists were able to come up with a list of measurements that are used to generate a profile of the molecule's conformation and the changes its structure undergoes.
The experiments were carried out in laboratory test-tubes using purified tau and HSP-90 molecules. The next step, says, Drescher, will be to repeat the measurements within living cells. The aim is "to observe the biochemical mechanism under the real-world conditions inside a cell." The team expects to make significant strides in understanding the world of AD from the inside out, and then to evolve methods to prevent this disorder.
Journal reference:
The mechanism of Hsp90-induced oligomerizaton of Tau, S. Weickert, M. Wawrzyniuk3,L. H. John, S. G. D. Rüdigerand M. Drescher, Science Advances 13 Mar 2020: Vol. 6, no. 11, eaax6999, DOI: 10.1126/sciadv.aax6999, https://advances.sciencemag.org/content/6/11/eaax6999