The whole world is waiting for one. A vaccine is the only thing that could conceivably save the world from a horrifying cascade of deaths, other than a miracle, it seems. With four countries (China, Italy, Spain and the USA) with highest numbers of reported cases, and two countries having overtaken China with approximately 10,779 and 6,803 deaths (Italy and Spain respectively) to China’s 3,300-plus deaths, the research establishment is frantically exploring all avenues to build a vaccine and induce immunity among thousands of vulnerable people.
However, the earliest time that a vaccine for COVID-19 hits the market in any useful volume is likely to be 1.5 years away. Every potential treatment, including vaccines, must undergo rigorous testing for safety and efficacy.
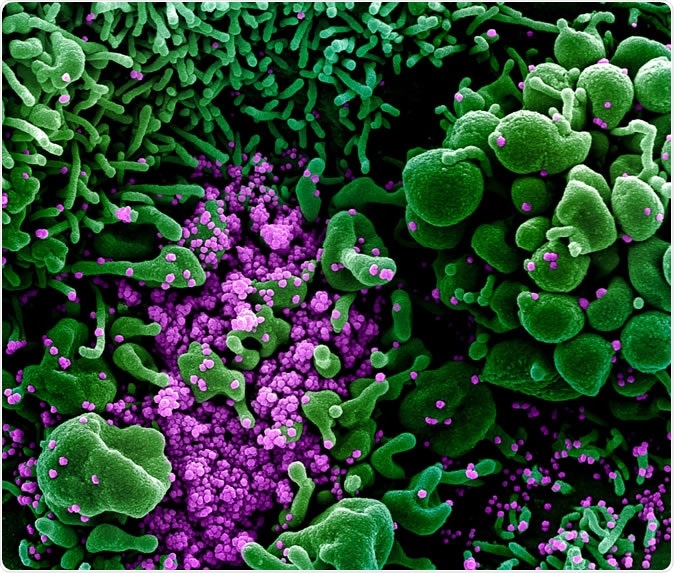
Novel Coronavirus SARS-CoV-2 Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
How to build a vaccine
One good thing about the virus that causes COVID-19 is that, unlike the flu virus, its genome shows little mutation. Thus, if and when a vaccine is prepared, it could remain effective for a long time.
The virus causing COVID-19 is a new one, and though its sequence is now widely published, it will take a long time and billions of dollars to convert this knowledge into a vaccine. In the most common methods of vaccine development, scientists use antigens – molecules that provoke an immune reaction by spelling harm or damage – on the target virus to create a look-alike that lacks the virus’s toxic capability but can produce a robust and precise immune reaction to the virus when it comes knocking.
The primary way to achieve this is to weaken the virus more and more until it is capable of little more than living and infecting a cell. Such an ‘attenuated’ virus is cultivated on animal cells (viruses cannot live or multiply outside living cells), extracted, and prepared in minute doses for use.
Another type of vaccine is the inactivated vaccine, which contains only specific viral proteins, typically those that enable the virus to invade human cells. When these are introduced into an animal, an immune response results, such vaccines tend to fade over time, making it necessary to give multiple doses to maintain immunity.
Finally, there are nucleotide-based vaccines, in which the genetic material from the virus is replicated as closely as possible. These are inserted into the body to stimulate the production of multiple copies of the viral DNA or RNA so as to promote the production of the necessary antibodies.
Stages of development
Most vaccines take anywhere from 5-15 years to reach the market. An exception was the nucleotide-based Zika vaccine. At present, 35 or more companies are racing each other to produce the first COVID-19 vaccine, with four front-runners having entered the experimental animal-testing phase. One is poised to enter the first human trials, but the results will take a long time.
According to infectious disease expert Anthony Fauci, vaccines can take at best 1-1.5 years to reach the market. A vaccine that you make and start testing in a year is not a deployable vaccine.” According to the Centers for Disease Control and Prevention (CDC), there are five stages of vaccine development:
- Exploratory stage, searching for potential compounds
- Preclinical testing in cells and then animals to find out more about how it works
- Clinical testing in three phases:
- Phase 1 is testing on a very few healthy adults to find out if it is safe enough to test
- Phase 2 is testing on more adults in the background of spreading disease, to test for the safety of use
- Phase 3 is testing on thousands of adults to find out if it is more effective than currently accepted
- Regulatory review and approval
- Manufacturing and quality control - finally, a vaccine molecule must be capable of production in large volumes.
Candidate vaccines
For instance, Norway-based CEPI, the Coalition for Epidemic Preparedness Innovations, is partnering with other organizations in the efforts to find a vaccine against COVID-19. Seeking $2 billion for research, the coalition wants to test more candidate vaccines upfront to arrive at three or more possible vaccines at the end. Many organizations working on the development of new vaccines include Inovio Pharmaceuticals, GlaxoSmithKline, and Moderna, which produce a DNA-based one, a molecular clamp that incorporates the protein by which the virus that causes COVID-29 enters human cells, and an RNA-based vaccine, respectively.
Inovio is the same company that produced a DNA-based vaccine for the Zika virus in just seven months, from design to the start of clinical trials. The RNA-based Moderna vaccine is the first experimental vaccine to enter human trials.
Artificial intelligence to the rescue?
To speed things up, some data analytics companies are introducing artificial intelligence (AI) to sift through mountains of big drug data to find those key patterns that indicate a possible vaccine candidate. Other investigators are focusing on repurposing older antiviral drugs and vaccines for use in COVID-19, such as an abandoned SARS vaccine of 2016. The key factor in these trials isn’t just money or time, but the number of current deaths occurring due to the lack of an effective vaccine, and secondarily due to the economic impact leaving irreversible damage
A prime issue separating ethical from non-ethical vaccine development is that of the level of acceptable risk, whether research can be done without consent and the importance of individual rights in the face of a pandemic. In the words of an informative piece in the Guardian, “So far, western countries have given greater priority to liberty and rights than eastern countries in responding to coronavirus, leaving them less able to secure public health. It is now, most likely, too late to eliminate the virus with isolation and quarantine. Scientific research looks the most promising option for a resolution to this pandemic.”