All over the world, except in China, the place where it all began, the COVID-19 pandemic is making its relentless way through the population. So far, it has caused nearly 34,000 deaths – and the situation in the US is still worsening with over 142,000 cases and 2,489 deaths. In Australia, the number of cases has climbed to 4,093, with 16 fatalities.
As healthcare systems gear up to meet the challenge of ten times as many patients without adequate critical care beds, personal protective equipment (PPE), or sufficient staff, various research initiatives are taking shape to protect those on the frontline. At present, there is neither a vaccine or medicine that can treat exposed healthcare workers effectively. Many of them are out sick or in quarantine, adding to the human toll as well as placing a greater strain on the healthcare services.
Thousands of doctors and other healthcare professionals are known to have been infected already in various parts of the world, and many have died. This is the inevitable outcome of repeated and close exposure to infected cases, often without proper PPE.
Testing the power of the BCG vaccine
But now, the Murdoch Children's Research Institute in Australia's Melbourne is preparing to begin a clinical trial to test whether the time-honored tuberculosis vaccine, BCG, will produce durable immunity against the SARS-CoV-2, better known as the novel coronavirus.
The BCG (Bacille Calmette-Guerin) vaccine is designed to protect against tuberculosis. First used in 1921, and is still administered to over 130 million infants around the world, in locations where tuberculosis is still rampant, for this purpose. However, the mechanism of action shows that it has a broader effect on human immunity, boosting the strength of the immune response to a number of new infections.
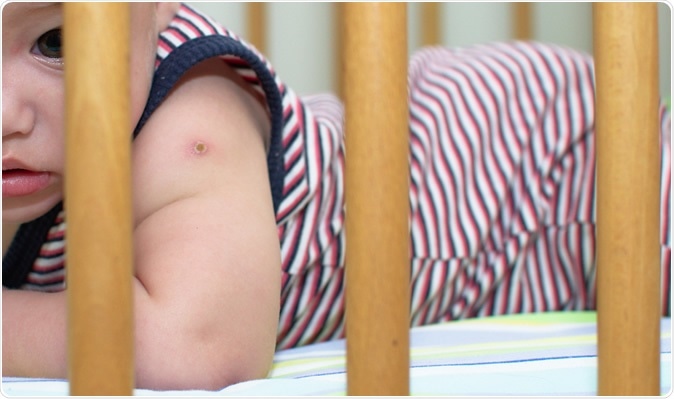
The red pop mark of BCG vaccine on the baby shoulder. Image Credit: Nym_Pleydell / Shutterstock
The unique immune-enhancing effects of the BCG vaccine are due to its ability to activate innate immunity, the body's first line of defense against intruders. This includes viruses and bacteria, which are met and disposed of before they even meet more specific immune barriers, in many cases. Having been used for over a century, its low incidence of severe side effects is a definite plus point. The most serious of them is the occurrence of a small granuloma at the site of injection, which fades into a small scar.
The BCG vaccine is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.
What are the unique benefits of BCG?
Researcher Professor Nigel Curtis points out that as a result of this activity, people who have had the vaccine are much more resistant to respiratory disease. After BCG immunization, people who do become infected by a virus show much lower viral loads in their blood compared to the unimmunized.
It is not, however, a specific antidote to the virus. Due to the eradication of tuberculosis in Australia, the BCG isn't currently available in the country. If the vaccine does work, it will be produced in large quantities to protect high-risk groups against the virus, such as the elderly.
Getting the vaccine will not alter or hinder the benefits of other medications that are currently being tried out against the virus, says Curtis. Virologist Professor Bill Rawlinson says, "It's a really great idea to trial alongside things like new antiviral medications and very important public health interventions that help prevent the spread."
The BRACE trial
The multicentre trial will be designed to meet the highest scientific standards as well as for the innovative use of existing vaccines to meet the COVID-19 threat. It will include 4,000 people working in various hospitals around the country and is planned to begin in the coming week if possible. Half of them will receive the vaccine, and differences between the groups will be assessed using an app.
The follow up will last six months and is intended to pick up early signs of COVID-19 and also to evaluate how severe the symptoms are in people who become infected following BCG vaccination.
The BRACE trial, as it is called, will build on earlier research indicating that BCG brings down the level of viruses similar to the SARS-CoV-2 in infected people. The hope is that this will result in fewer and less intense symptoms of COVID-19 in healthcare workers who are vaccinated with BCG.
Speaking of the generous gifts that have made this rapid research effort possible, Professor North says, "Using rapidly sourced and immediately deployable funds, these trials will allow the rapid advancement of the most promising candidates to clinical practice, giving us the most number of shots on goal against COVID-19 as possible."
Similar trials are ongoing in Germany, Spain, and the US.
What will the BRACE trial prove?
Researcher Professor Kathryn North A. C. says, "This trial will allow the vaccine's effectiveness against COVID-19 symptoms to be properly tested, and may help save the lives of our heroic frontline healthcare workers." In fact, they say it could be useful as an "off-the-shelf vaccine that works against different viruses" to protect against widespread infection in future pandemics as well. And, says Professor Curtis grimly, "This has really alerted to the world that we are always just a few weeks away from a pandemic."
Repurposing an older drug
Another approach is medication. An antiviral drug called remdesivir initially developed to fight the outbreak of Ebola in 2009, but not pressed into service because of its ineffectiveness, has gained a potential new lease of life. The drug is being trialed across the world in several clinical settings, from a 79-year-old Italian man who was confirmed to have COVID-19, was treated with remdesivir, and subsequently had a negative test, to 13 patients who were infected onboard the cruise ship Diamond Princess. The Italian was described by politician Giovanni Toti as the "first real case of coronavirus cured."
Remdesivir is also one of the drugs being tested in the massive trial being initiated by the World Health Organization (WHO) to test out the four most promising drugs for the treatment of COVID-19 – which also includes malarial drugs chloroquine and hydroxychloroquine, and the HIV medications lopinavir and ritonavir.