.jpg)
Novel Coronavirus SARS-CoV-2: This scanning electron microscope image shows SARS-CoV-2 (round gold objects) emerging from the surface of cells cultured in the lab. SARS-CoV-2, also known as 2019-nCoV, is the virus that causes COVID-19. The virus shown was isolated from a patient in the U.S. Credit: NIAID-RML
As well as potentially providing useful information for antibody therapy and vaccine development, the findings could help ensure that community testing is timely and sensitive enough to be effective and reliable.
About the pandemic
Since its outbreak in December 2019, the spread of COVID-19 has become a global public health threat. As of today, April 13th, the SARS-related coronavirus – SARS-CoV-2 – has caused 1,911,407 reported infections and 118,854 deaths.
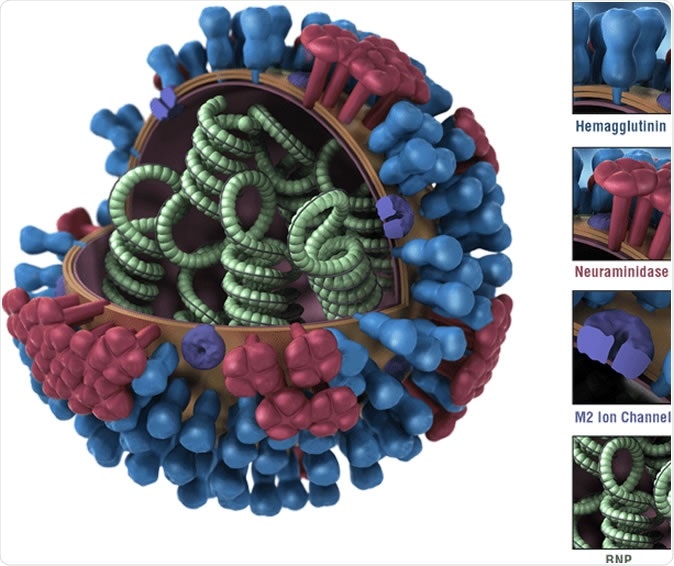
Diagram of an influenza virus. Influenza A viruses are classified by subtypes based on the properties of their hemagglutinin (H) and neuraminidase (N) surface proteins. There are 18 different HA subtypes and 11 different NA subtypes. Subtypes are named by combining the H and N numbers – e.g., A(H1N1), A(H3N2).
Most infected individuals only experienced mild symptoms, but some 14% progressed to more severe disease characterized by symptoms such as difficulty breathing and low blood oxygen saturation. In around 5% of cases, the condition became critical, particularly among those aged 60 years or older or those with existing health problems, and approximately 3.4% died from organ or respiratory failure.
Virus-specific NAbs
The development of virus-specific NAbs either as a result of infection or immunization is known to block, and clear viral infections, and researchers expect that (NAbs) against SARS-CoV-2 contribute towards disease protection and patient recovery.
Passive antibody therapy using plasma or serum from recovered donors has previously been shown to treat infections such as influenza, Ebola, and SARS-CoV, and its efficacy has been correlated with donors’ levels of NAbs or other antibodies. This therapy has been considered a potential approach to preventing and treating COVID-19, yet SARS-CoV-2-specific NAbs have not yet been well studied among COVID-19 patients with clinical symptoms.
Now, Jinghe Huang and colleagues have used a sensitive, safe, and reproducible neutralization assay to screen for levels of SARS-CoV-2-specific NAbs in plasma samples taken from 175 COVID-19 patients who had recovered from mild disease.
“Herein, we aimed to explore the clinical characteristics associated with the level of NAbs in recovered patients, the outcome of which may provide useful information for the development of vaccines and passive antibody therapy for the prevention and treatment of SARS-CoV-2,” writes the team.
Although the paper (available on MedRxiv) is currently at the pre-print stage and has not yet been peer-reviewed, Eleanor Riley, Professor of Immunology and Infectious Disease at the University of Edinburgh says it “is a well written and methodologically sound study,” and “provides a very useful set of data.”
The main findings
Key findings were that most patients developed SARS-CoV-2-specific NAbs during the early stage of COVID-19 disease. NAb levels peaked between 10 and 15 days following disease onset, after which they remained stable.
Professor in Medicine at the University of East Anglia, Paul Hunter, says this “provides further evidence that if we do manage to roll out antibody tests, people should wait about two weeks after becoming ill to test themselves.”
However, approximately 30% did not develop high levels of NAbs, yet disease duration among these patients did not significantly differ, compared with patients who did develop high levels.
“This has relevance to the current debate about antibody tests in the UK,” says Hunter. “If many people only produce low levels of antibodies to SARS-CoV-2, then any community test would need to have high sensitivity. This provides further insight into why community antibody tests in the UK have not yet been authorized for use.”
Furthermore, for ten patients, NAbs were not detectable at all, indicating that other immune system factors such as cytokines or T cells may have contributed to recovery, suggest the researchers:
“Whether these patients were at high risk of rebound or reinfection should be explored in further studies,” they say.
Elderly individuals may have had a stronger innate immune response
The team also reports that among those who did develop NAbs, levels were significantly higher among elderly patients than among younger patients and correlated with markers of disease severity. Elderly patients had higher blood CRP levels, and lower lymphocyte counts on admission to the center, suggesting a stronger innate immune response than among younger patients.
“Whether the high level of NAbs protects these patients from progression into severe and critical conditions is worthy of a comprehensive evaluation,” writes the team.
The findings “should be further explored” for vaccine development
The authors say that to the best of their knowledge, their study is the first to report on plasma NAbs among patients who have recovered from COVID-19.
“The correlation of NAb titers with age, lymphocyte counts, and blood CRP levels suggested that the interplay between virus and host immune response in coronavirus infections should be further explored for the development of an effective vaccine against SARS-CoV-2 virus,” concludes the team.
Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Sources:
Journal reference: