The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has challenged hospitals and laboratory services worldwide. Diagnostic testing for SARS-CoV-2 initially lagged but is now primarily based on the use of reverse transcriptase-polymerase chain reaction (RT-PCR) to detect viral nucleic RNA in the samples. However, viral RNA has also been reported in several small series of cases, though its significance and frequency remain unclear.
SARS-CoV-2 viruses binding to ACE-2 receptors on a human cell, the initial stage of COVID-19 infection, conceptual 3D illustration. Credit: Kateryna Kon / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
What is the PCR Test?
The PCR test is based upon the principle of amplifying, or increasing the amount of, the tiny amounts of viral nucleic acid typically present in a biospecimen, whether from the throat, nose or nasopharynx. While it is mostly used to identify the presence of SARS-CoV-2 nucleic acid in these locations, it is also possible to pick up viral RNA from these and other sites.
Several small studies have reported the finding of viral RNA from blood, serum, and plasma. However, it is essential to find out what this actually implies in terms of the presence of infectious viral particles in these samples. If there is a sound correlation, the occurrence of PCR-positivity in these locations could indicate the severity and outcome of the disease. Moreover, it could dictate the type of safety norms that must be followed by clinical and laboratory workers in both routine and COVID-19-specific testing.
The various sets of guidelines now being followed are not backed by experience and use the presence of viral RNA as an indicator of the presence of live virus particles. This, in turn, requires viral inactivation protocols, which add time and complexity as well as increased costs to the testing process. Moreover, they could reduce the sensitivity of the tests, which is detrimental to the outcome of serological assays and antibody detection in particular.
If this is not done, a biosafety level 3 facility is the only viable option. This is unrealistic, given the cost of establishing and maintaining such a laboratory, the specialized training required, the significantly fewer number of samples that can be thus processed, and the relative inaccessibility of such centers. The fundamental uncertainty about the kind of precautions necessary to be taken in such a setting leads to unnecessary escalation to this level of biosafety.
The current study aims to review available literature to provide data that may help understand why and how viral RNA is present in blood. In addition, the investigators examined 424 blood samples from acutely infected and convalescent patients with COVID-19 to look for viral RNA. They tried to isolate live viruses from these cultures to determine the importance of RNA as a marker of the live virus.
How Was the Study Done?
The researchers evaluated 22 studies to find the prevalence of viral RNA detectable in blood and the correlation of this finding with disease manifestations.
They then collected 429 samples from 384 individuals as follows:
- 94 patients gave 139 serum samples between 1-5 days of admission to either hospital or intensive care, at a median of 8 days from the earliest symptoms
- 41 samples from 41 convalescent workers at a median of 7 days from symptom onset
- 32 samples from 32 convalescent patients at a median of 42 days from symptom onset
- 212 samples from 212 convalescent plasma donors at 28 or more days from symptomatic recovery
- 5 samples from 5 healthy pre-pandemic donors
RT-PCR was carried out on the serum sample nucleic acid. Twenty samples from both acute and convalescent samples were selected at random for viral culture.
What Did the Study Show?
The meta-analysis of 22 studies showed that there was no agreement on the extent of RNA detection in blood, which ranged from 0% in some studies to 76% in one report. The point estimate is 10% for RNA in blood specimens within 28 days of symptom onset.
Some researchers reported a higher level of viral RNA in blood with more severe disease, but not others.
Of the 167 individuals whose blood samples were tested (some samples were duplicated), there were 20 with viral RNA in blood. Multivariate analysis indicates that finding viral RNA in blood is linked to critical illness. Using a time-dependent pooled sample, they found that 23/131, or about 18%, had viral RNA in blood up to the 13th day, 4/40 or 10% on days 14-27, and none beyond that. In fact, no PCR positivity was detected beyond day 20.
If the standard cycle threshold values used in most clinical laboratories to define a positive result, only 7/25 results would have been positive.
Further testing for the presence of live infectious virus failed to show significant results.
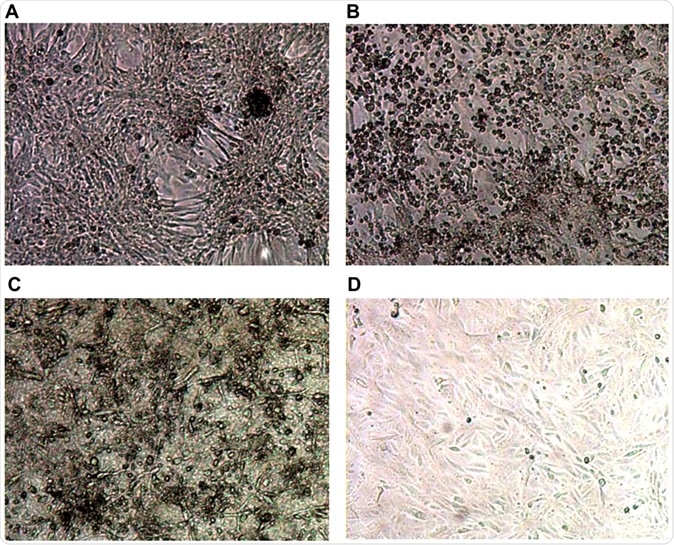
Typical images from cell culture in an in vitro system for SARS-CoV-2 culture. Top row shows controls: (A) Negative control Vero E6 cells in media; (B) Cytopathic effect (CPE) in Vero E6 cells spiked with Victoria/01/2020 SARS-CoV-2; Bottom row shows Vero E6 cells inoculated with 1/10 dilution of serum sample from sample VC12 (patient ID UKCOV040), that tested positive for SARS-CoV-2 RNA by RT-PCR; (C) Aberrant cellular effects at day 4 in a culture inoculated with VC12 at day 0; (D) Normal appearance of cells at day 7 inoculated with 1/10 dilution of the culture supernatant of the VC12-challenged culture, illustrated in (C).
How is the Study Important for COVID-19 Management?
The results show that viral RNA may be present at very low levels in about a tenth of blood samples from COVID-19 patients before day 28. Most RNA-containing samples were collected earlier in the course of the illness and from more severely ill patients. Not even one showed the presence of infectious viral particles.
The findings agree with a previous study showing that even in severe illness, RNA is found in blood in 45% of cases at the time of admission but only 11% by week 4. In mild cases, the decline is from 27% to none at the same time points. Thus, the detection of viral RNA may indicate severe or critical disease, either due to higher viral load or because of an overabundance of viral particles in the lungs spilling over into the bloodstream. Alternatively, it could indicate the destruction of infected cells in the respiratory lining.
More research is required to understand which of these explanations is right, and whether this RNA in peripheral blood can induce immune dysregulation and systemic hyperimmune response, along with acute respiratory distress syndrome.
The study concludes: “We have found no evidence to suggest that blood samples containing RNA could yield replication-competent virus, suggesting a negligible risk of transmission of SARS-CoV-2 to healthcare workers and laboratory staff from handling such material.” Nonetheless, public health guidelines are recommended as well as the evaluation of individual risk depending on the type of infectious material and the particular test being done. In all cases, universal precautions are necessary to prevent not only COVID-19 but other infections.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources