By combining T and B lymphocyte analysis with manufacturability heuristic, researchers from the US and Germany proposed a set of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine peptides to use in subsequent animal studies and clinical trials. Their paper is currently available on the bioRxiv* preprint server.
The current pandemic of coronavirus disease (COVID-19), caused by the SARS-CoV-2 virus, has infected millions of people and resulted in hundreds of thousands of deaths. As treatment and management options are still limited, the development of an efficient vaccine is pivotal for mitigating public health impact.
Similar to SARS-CoV-1 that caused the SARS outbreak, SARS-CoV-2 enters human cells via interaction of the viral receptor-binding domain (RBD) with angiotensin-converting enzyme 2 (ACE2) receptors, which are pervasive on the surface of human nasopharyngeal, lung and intestinal mucosa.
Consequently, the production of neutralizing antibodies that target RBD or other functional domains is thought to be essential for vaccine efficacy. Thus far, SARS-CoV-2 vaccines have primarily focused on generating B lymphocyte (or B cell) responses to trigger the production of neutralizing antibodies.
And while antibodies against SARS-CoV-2 have been identified in COVID-19 patients, it is still unknown which of these antibodies actually drive viral neutralization, antibody-dependent enhancement of infectivity that makes the infection even more acute, or possibly both.
Therefore, vaccine efficacy and safety have to be optimized by innovative approaches that increase the generation of neutralizing antibodies, while at the same time minimize antibody-dependent enhancement or pulmonary immune pathology.
Vaccine strategies
Current vaccine strategies for tackling SARS-CoV-2 include recombinant spike glycoprotein (S protein), nucleic acid encodings of the S glycoprotein, recombinant RBD, adenovirus vector/live recombinant measles vaccine expressing the S protein, as well as the delivery of whole inactivated virus.
Multi-epitope peptide vaccines represent an alternative approach that has a history of safe administration, rapid development and update, and less risk for eliciting non-neutralizing antibodies that contribute to antibody-dependent enhancement.
This is why researchers from the UNC School of Medicine (North Carolina), Icahn School of Medicine at Mount Sinai (New York), University of California (Santa Cruz), University of Georgia (Athens), as well as PEPperPRINT GmbH (Heidelberg, Germany) reported a comprehensive survey of the T and B cell epitope spaces of SARS-CoV-2.
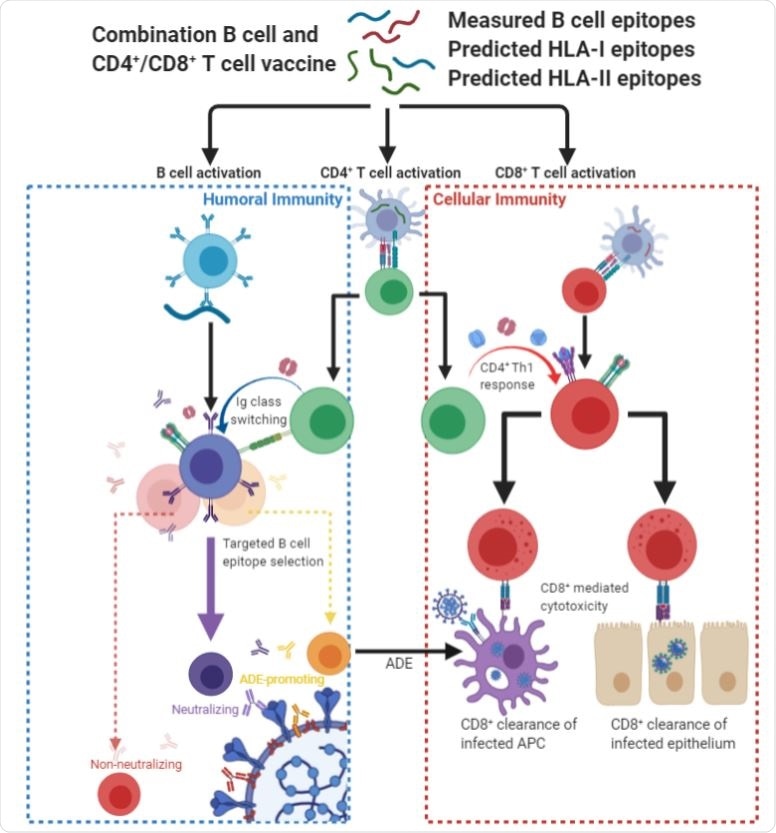
"We hypothesize that peptide vaccines containing epitope regions optimized for concurrent B cell, CD4+ T cell, and CD8+ T cell stimulation would drive both humoral and cellular immunity with high specificity, potentially avoiding undesired effects such as antibody-dependent enhancement", explain the authors of the study available on bioRxiv.
Taking a walk in the epitope landscape
In this study, the researchers combined computational forecasting of T cell epitopes, recently published B cell epitope mapping studies, as well as epitope accessibility in order to hand-pick peptide candidate vaccines for SARS-CoV-2. The result is a survey of the SARS-CoV-2 epitope landscape along with a prioritization strategy for vaccine development.
The study began with an investigation of the possible T cell epitope locations in SARS-CoV-2 with the appraisal of predicted histocompatibility complex of class I and II ligands (and overlap between them), protein source, as well as simultaneous human/murine coverage.
Beyond the affinity for the major histocompatibility complex (MHC) that plays a crucial role in the adaptive branch of the immune system, T cell vaccine candidates were further refined by predicted immunogenicity, sequence conservation, viral source protein abundance, and co-localization of epitopes.
Furthermore, B cell epitope regions were chosen from linear epitope mapping studies of convalescent patient sera, which was followed by filtering to select regions with surface accessibility, spatial localization next to functional domains of S protein, high sequence conservation and avoidance of glycosylation sites.
From 58 initial candidates, three pertinent B cell epitope regions were identified near solvent-exposed surfaces of the S protein. This research group decided to focus on conserved regions of the virus to pinpoint epitopes that would be most broadly targetable in humans.
"No other study to date has considered all such features in their epitope selection process," explains study authors. "Additionally, the inclusion of corresponding murine epitopes allows for future studies to be performed in animal models of SARS-CoV-2", they add.
A part of the multifaceted response
In a nutshell, a peptide vaccine targeting B lymphocytes, CD4+ T lymphocytes and CD8+ T lymphocytes in parallel may prove an indispensable part of a multifaceted response to the COVID-19 pandemic.
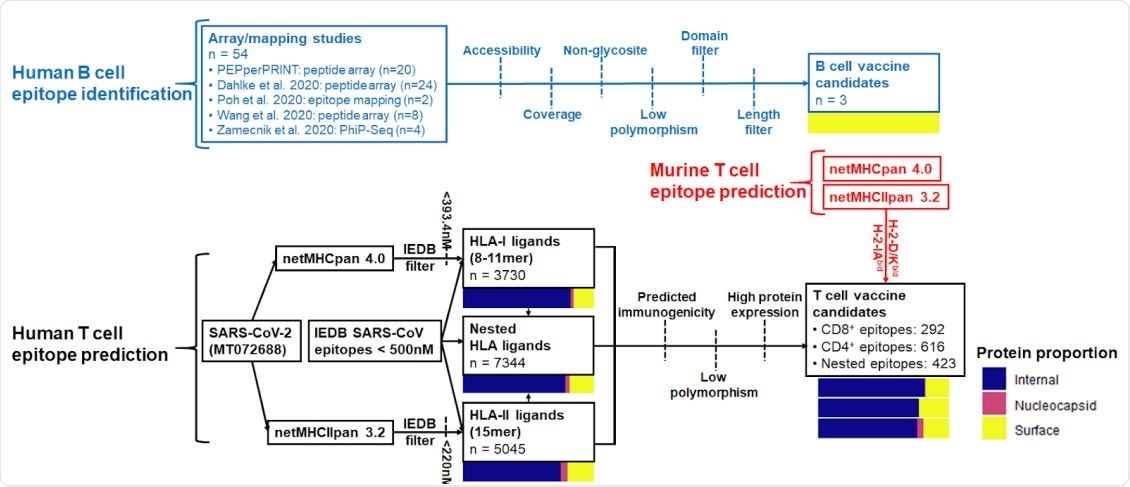
Summary of B cell and CD4+ /CD8+ epitope prediction workflows. Pathways are colored by B cell (blue), human T cell (black), and murine T cell (red) epitope prediction workflows. Color bars represent proportions of epitopes derived from internal proteins (ORF), nucleocapsid phosphoprotein, and surface-exposed proteins (spike, membrane, envelope).
More specifically, such an approach has a potentially advantageous development timeline and the propensity to avoid antibody-dependent enhancement by precisely steering the immune response toward functional (i.e., neutralizing) regions.
However, it must be emphasized that epitope selection represents only one important facet of this problem, and a fundamental question is whether a peptide vaccine can be sufficiently immunogenic for the recipient.
Hence, adjuvant selection, conjugation to carriers, and prime/boost approaches by using orthogonal platforms are potential avenues to explore. The sets of vaccine peptides reported in this study may prove of great value during the preclinical development of these approaches.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources