Researchers from France and the U.S. demonstrated how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection could induce an early and vigorous virus-specific IgA response that paves the way for the production of SARS-CoV-2 IgG antibodies, with even better neutralizing properties. Their paper is currently available on the medRxiv* preprint server.
Antibodies, 3D rendering - Illustration Credit: ustas7777777 / Shutterstock
The ongoing pandemic of coronavirus disease (COVID-19), caused by SARS-CoV-2, has taken a significant toll on many countries worldwide, infecting more than 7.84 million people as of June 15.
In response to infectious agents, the human immune system produces five major types of antibodies: IgG, IgM, IgA, IgE, and IgD. A major dogma in immunology states that the IgM antibody response actually precedes secondary memory responses built on the production of IgG, IgA, and (occasionally) IgE antibodies.
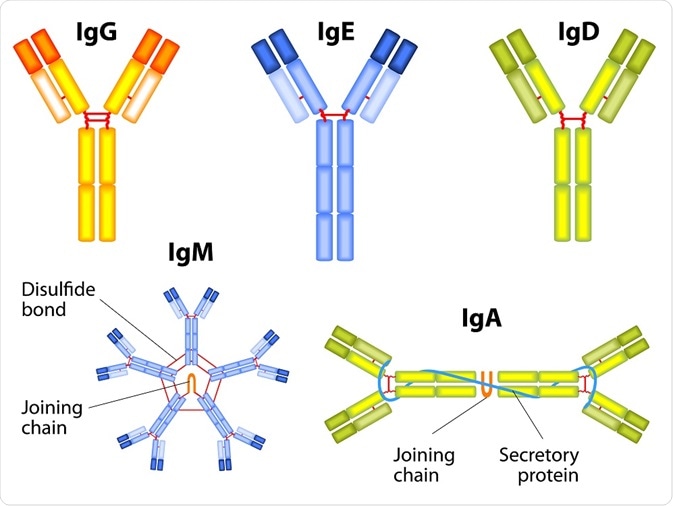
Designua | Shutterstock
Recent research reports specify that SARS-CoV-2 generates robust antibody responses, including specific IgG, IgA, and IgM. Seroconversion in infected individuals occurs within 20 days after symptom onset, albeit with disparate kinetics of IgM and IgG production.
Generally speaking, secretory IgA plays a pivotal role in safeguarding mucosal surfaces against pathogens by neutralizing respiratory viruses or hindering their attachment to epithelial cells; hence, it may play an important role in coronavirus infections as well.
Accordingly, recent interventions based on an intranasal challenge with SARS proteins or MERS-derived vaccine confirmed the beneficial role of IgA in coronavirus infections. Nonetheless, to which extent IgA production intervenes to control natural SARS-CoV-2 infection in humans is still poorly understood.
A research group from Sorbonne Université, Assistance Publique Hôpitaux de Paris, Institut Pasteur, and Theravectys in France, as well as from Genalyte Inc. in the U.S., decided to track antibody-secreting cells in the blood of SARS-CoV-2-infected patients. They also aimed to determine specific serum antibody titers and to study their neutralizing capacities.
Appraising neutralization capacity
The crux of their methodological approach was to measure acute humoral responses to SARS-CoV-2 – Including the occurrence rate of antibody-secreting cells and the presence of specific, neutralizing antibodies in serum and bronchoalveolar fluid of 145 COVID-19 patients.
Flow cytometry (i.e., a popular biology technique with laser-based technology to count, sort, and profile cells) was employed to longitudinally monitor phenotypic changes of B lymphocytes (antibody-secreting cells) in the blood of patients with COVID-19.
Moreover, by using photonic ring immunoassay – a novel technology that allows simultaneous testing of various antigens in parallel – the researchers assessed the prevalence of IgG, IgA, and IgM antibodies recognizing the SARS-CoV-2 full-length nucleocapsid protein or spike receptor-binding domain.
"We then sought to determine the respective contribution of each of the IgG and IgA isotypes to virus neutralization," study authors further explain their methodological approach. "We assessed the neutralizing capacity of serum antibodies using a pseudoneutralization assay," they add.
Rapid response, but also a swift decline
"Our results show that human IgA antibodies are often detectable before the appearance of SARS-CoV-2-specific IgG and argue in favor of a key role for IgA antibodies in early virus neutralization", study authors emphasize their main finding.
Re-circulating plasmablasts (i.e., immature antibody-secreting cells typical for the acute phase of viral infection) that secrete IgA antibodies and have a mucosal-homing potential were detected in high numbers shortly after symptom onset, and they peaked during the third week of the disease.
However, a rapid decline in SARS-CoV-2-specific IgA serum levels was detected (already after one month), questioning, in turn, the long-term feasibility of this first wave response, as efficient as it seems to be.
Furthermore, some of the early human sera that had an efficient virus neutralizing capacity revealed only anti-RBD IgM spike specific antibodies above the detection threshold, but not IgA nor IgG – pointing towards the protective potential of IgM antibodies as well.
Inducing specific respiratory IgA response as a way forward?
A contribution of IgA to viral neutralization to a much larger extent when compared to IgG represents a challenging discovery, given the current uncertainty as to which kind of specific humoral response would optimally protect against re-infection.
Still, we need to know whether the prevalence of IgG in bronchoalveolar samples is representative of the pulmonary humoral status in mildly affected and convalescent patients, as the ones tested in this study were all from severe COVID-19 cases with serious lung damage and possible serum contamination.
Therefore, to adequately address this issue, future studies should evaluate the presence of SARS-CoV-2-specific secretory IgA antibodies in easily attainable samples, such as saliva from COVID-19-recovered patients.
Also, longitudinal studies are necessary at various body sites in order to appraise whether local SARS-CoV-2-specific IgA production might be more persevering and reliable than in blood.
"In conclusion, we would like to stress the importance of mucosal immunity as an important defense mechanism against SARS-CoV-2 to be monitored in infected patients, as well as to recommend testing the usefulness of a vaccine protocol aimed at inducing a specific respiratory IgA response to SARS-CoV-2", highlight study authors.
It remains to be seen whether the in vitro IgA neutralization efficacy of the purified IgA serum antibodies (as seen in this study) can translate into a potent barrier effect – not only in recovered patients but also in healthy carriers and those with low symptom burden.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources