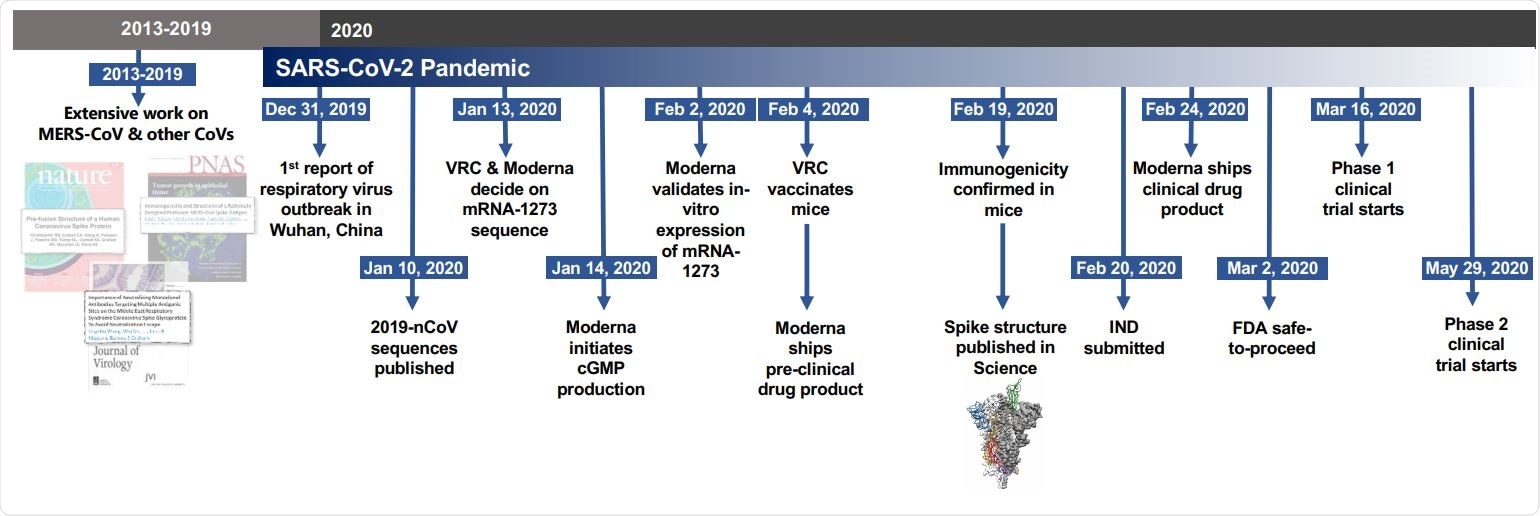
Timeline for mRNA-1273’s progression to clinical trial. The morning after novel coronavirus (nCoV) sequences were released, spike sequences were modified to include prefusion stabilizing mutations and synthesized for protein production, assay development, and vaccine development. Twenty-five days after viral sequences were released, clinically-relevant mRNA-1273 was received to initiate animal experiments. Immunogenicity in mice was confirmed 15 days later. Moderna shipped clinical drug product 41 days after GMP production began, leading to the Phase 1 clinical trial starting 66 days following the release of nCoV sequences.
Barney Graham and colleagues report on the protective neutralizing and CD8 T cell responses induced by the vaccine, which is hoped to progress to phase 3 efficacy evaluation eventually.
The team developed the vaccine based on the atomic-level structure assessment of betacoronavirus Spike proteins, which guided the application of mutations to improve the proteins’ expression and immunogenicity. Spike proteins are the surface membrane structures that the viruses use to bind to and enter host cells.
The authors say the findings provide a proof-of-concept for a new “preparedness” approach to the threats future viruses could pose.
A pre-print version of the paper can be accessed in the server bioRxiv*, while the article undergoes peer review.
.jpg)
Novel Coronavirus SARS-CoV-2 Transmission electron micrograph of a SARS-CoV-2 virus particle, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
Vaccines are “critical for changing the global dynamic of this virus”
Since coronavirus disease 2019 (COVID-19) was first identified in Wuhan, China, late last year, its causative agent - SARS-CoV2 - has infected more than 7.84 million people and caused more than 431,000 deaths.
It is estimated that the pandemic will not be brought under control enough for normal activity to resume until 60 to 70% of the population has developed an immunity. However, if that immunity relies on infection only, more than 40 million people would become infected in the meantime.
“Therefore, the rapid development of vaccines against SARS-CoV-2 is critical for changing the global dynamic of this virus,” writes Graham and colleagues.
Prefusion-stabilized protein immunogens
On binding to a host cell receptor, the Spike protein undergoes a significant structural rearrangement that enables fusion with the host cell membrane and delivery of viral cargo into target cells.
Graham and the team previously demonstrated that prefusion-stabilized protein antigens that contain neutralization-sensitive epitopes serve as an effective vaccine approach against enveloped viruses.
The team identified two proline substitutions (2P) in the Spike (S) proteins of SARS-CoV-1 and the Middle East respiratory syndrome-CoV (MERS-CoV) that stabilized them in the prefusion conformation. The researchers found that the MERS S-2P protein was transferable to many other betacoronavirus S proteins, suggesting the strategy could be broadly applied in the design of stabilized prefusion beta-CoV S immunogens.
Nucleic acid vaccines are quicker to manufacture
The rapid development of a vaccine in response to a newly emerged virus requires precise structure-based immunogen design and an agile manufacturing platform that will accelerate the time to product availability. This can take more than a year if cell lines and protein subunits are used, while nucleic acid vaccines can be manufactured in a matter of weeks.
As well as mRNA vaccines being quick to manufacture, they are also highly immunogenic and induce both innate and humoral immune responses.
The researchers, therefore, assessed mRNA formulated in lipid nanoparticles (mRNA/LNP) to deliver MERS-S-2P to mice. They found that transmembrane-anchored MERS S-2P mRNA induced more potent neutralizing antibody responses than secreted MERS S-2P and was more immunogenic than wild-type MERS S mRNA.
Making the 2P substitutions in SARS-CoV-2 Spike protein
Soon after its identification in Wuhan in early January, the SARS-CoV-2 isolate sequences were released, and within 24 hours, Graham and colleagues had applied 2P substitutions to make a prefusion-stabilized SARS-CoV-2 S-2P protein.
The team then produced mRNA/LNP expressing SARS-CoV-2 S-2P as a transmembrane-anchored protein with the native furin cleavage site (mRNA-1273) and evaluated its effects in six-week-old mice.
Administration of a single dose of 1 or 10 µg of mRNA-1273 generated S-binding antibodies, with the 10 µg dose also inducing an increase in neutralizing antibody activity between two to four weeks later.
“These data demonstrate that mRNA expressing SARS-CoV-2 S-2P is a potent immunogen and neutralizing activity can be elicited with a single dose,” writes the team.
Furthermore, a 1 µg does of mRNA-1273 elicited a robust CD8+ T cell response to the S1 subunit of the Spike protein.
The researchers also challenged mice that had received two 1 μg doses of mRNA-1273 with mouse-adapted SARS-CoV-2 that replicates in the lungs.
“Mice were completely protected from viral replication in the lungs after challenge at 5- or 13-week intervals following boost,” reports the team.
“Robust neutralizing activity”
“Here, we showed that 1 μg of mRNA-1273 was sufficient to induce robust neutralizing activity and CD8+ T cell responses, and protection from viral replication for more than 3 months following a prime/boost regimen similar to that being tested in humans,” said Graham and colleagues.
The researchers say these findings, together with data from nonhuman primates and participants in phase 1 clinical trials, will help to inform the dose and regimen of mRNA-1273 in later-stage clinical efficacy trials.
“It is also a proof-of-concept for the prototype pathogen approach for pandemic preparedness and response that is predicated on identifying generalizable solutions for medical countermeasures within virus families or genera,” says the team. “There are 24 other virus families known to infect humans, and with a sustained investigation of those potential threats, we could be better prepared for future looming pandemics.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources