Resolving the damage of coronavirus disease (COVID-19) pandemic to health care, the economy, and society as a whole will necessitate the distribution and testing scale-up to unprecedented levels. Consequently, there is widespread interest in developing alternatives to nasopharyngeal swabs and other sampling methods currently available for COVID-19 diagnosis.
Among these alternatives is the collection of nasal swab samples, which can even be performed by the patient, eliminating the need for healthcare professionals and personal protective equipment. However, previous studies appraising their use had come to opposite conclusions, e.g., when nasal sampling was compared to nasopharyngeal swabbing.
To address this important issue, the researchers from Beth Israel Deaconess Medical Center and Harvard Medical School in Boston, US, have decided to compare two different healthcare-worker nasal-swab collection procedures and three different transport conditions.
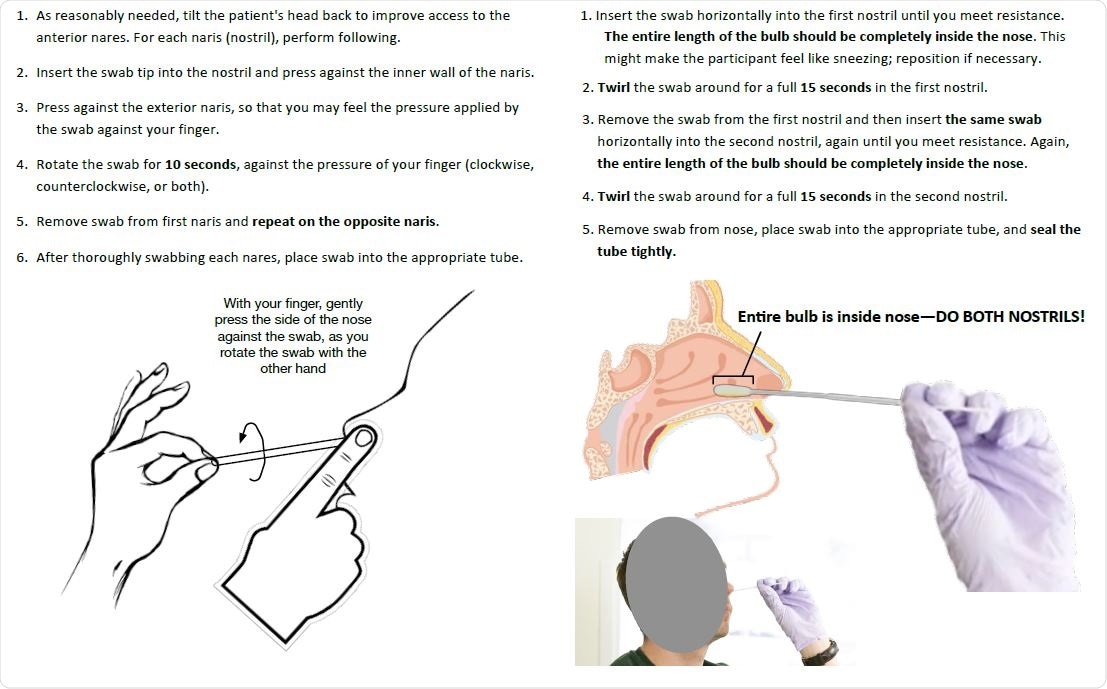
Nasal swabbing instructions
A minimally-invasive sampling approach
In short, these researchers devised their study protocol as a six-arm study with a total of 308 participants. All of the study subjects were adults older than 18. They were tested for SARS-CoV-2 during the normal course of clinical care (either due to clinical suspicion of COVID-19 infection or as a follow-up after previous SARS-CoV-2-positivity).
Standard nasal swabs were compared under three diverse specimen-transport conditions: a guanidine thiocyanate transport buffer, in modified CDC viral transport media, and with no buffer at all. Reverse transcription-polymerase chain reaction (RT-PCR) has been used for identification and quantification purposes.
When taking a nasal swab for each nostril, the patient was instructed to press a finger against the exterior of that nostril upon insertion of the swab tip, while the swab was rotated against this external pressure for ten seconds. The nasopharyngeal swabs were collected from a single nostril by standard method.
Nasal swabs reliable for high viral loads
"Our results strongly suggest that concordance between nasal and nasopharyngeal swabs depends on the limit of detection of the PCR assay used to measure positivity, with a high concordance for medium-to-high viral loads and low concordance for low viral loads, which may lie below the limits of detection of various assays currently in widespread use", explain study authors their main findings.
More specifically, they have found that nasal swab samples can reliably detect patients with viral loads high than 1,000 copies per milliliter of human blood but may miss many patients who have lower viral loads – which were actually the majority of patients in this study.
Importantly, based on viral load distribution in individuals that were tested for the first time in this study, approximately 20% of those infected with SARS-CoV-2 would be missed if sampled using just nasal swabs, emphasizing the extent of this problem.
"A complete biological explanation will require further study; however, one possibility is that in cases of high viral load, replicating virus may be more likely to spread to respiratory epithelium bordering and/or in the deeper portions of the anterior nares, where it can be recovered by nasal swab", say study authors.
Conversely, no differences were noted between sampling protocols and among transport media conditions, which suggests that the observed lower sensitivity of nasal swab sampling represents a universal limitation of the anatomical location and that the protocols and media conditions tested in this study are interchangeable. This is good news, as it gives us a myriad of potential options for specimen acquisition.
Pros and cons for using nasal swabs in SARS-CoV-2 testing
There are considerable advantages of nasal swabs in terms of collection simplicity and the possibility of self-collection. Based on the results of this study, their role would be optimal in high volume, prevalence screening endeavors in healthy populations (such as universities and working places), where it would basically serve as a prelude to targeted testing with more sensitive methods.
On the other hand, nasal swabs should not be utilized for screening symptomatic and hospitalized patients; this is the population of particular interest where much more sensitive and resource-intensive nasopharyngeal sampling is definitely justified to aid in directing care and infection control resources.
Overall, this study suggests to be extremely cautious with the use of nasal testing in the healthcare arena, but also all contact-tracing efforts, and warns that nasal testing should be seen as an adjunct and not a replacement for nasopharyngeal swabs.
In other words, while nasal swabs are welcome as an additional 'weapon' in combating COVID-19, all healthcare workers should be informed and well aware of the aforementioned limitations that may hamper its diagnostic sensitivity. Therefore, this resource should be used carefully and sagaciously.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources