Researchers at Columbia University, Dana-Farber Cancer Institute, and the National Institutes of Health have identified and characterized a range of monoclonal antibodies that potently neutralize severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Image Credit: Shutterstock/Mirror-Images

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The team says the collection of monoclonal antibodies they have isolated is unprecedented since previous ones have so far been much less potent.
They also say several of the antibodies are promising candidates for the development of preventive or therapeutic approaches to combatting the virus.
A pre-print version of the paper is available on the server bioRxiv*, while the article undergoes peer review.
Developing solutions to help resolve the COVID-19 pandemic
As the coronavirus disease 2019 (COVID-19) pandemic continues to sweep the globe disrupting lives and devastating the economy, scientists are working around the clock to find solutions that might help enable a return to normality.
Drug development is rapidly progressing, and several vaccines are now being tested in clinical trials.
Another approach has been the search for monoclonal antibodies (mAbs) that could prevent or treat infection, the primary target of which is the viral Spike protein that binds to ACE2 expressed on host cells.
This Spike protein is made up of two subunits. The S1 subunit has a receptor-binding domain (RBD), and an N-terminal domain (NTD) and the S2 subunit mediates fusion of the virus membrane to the host cell, once the RBD has attached to ACE2.
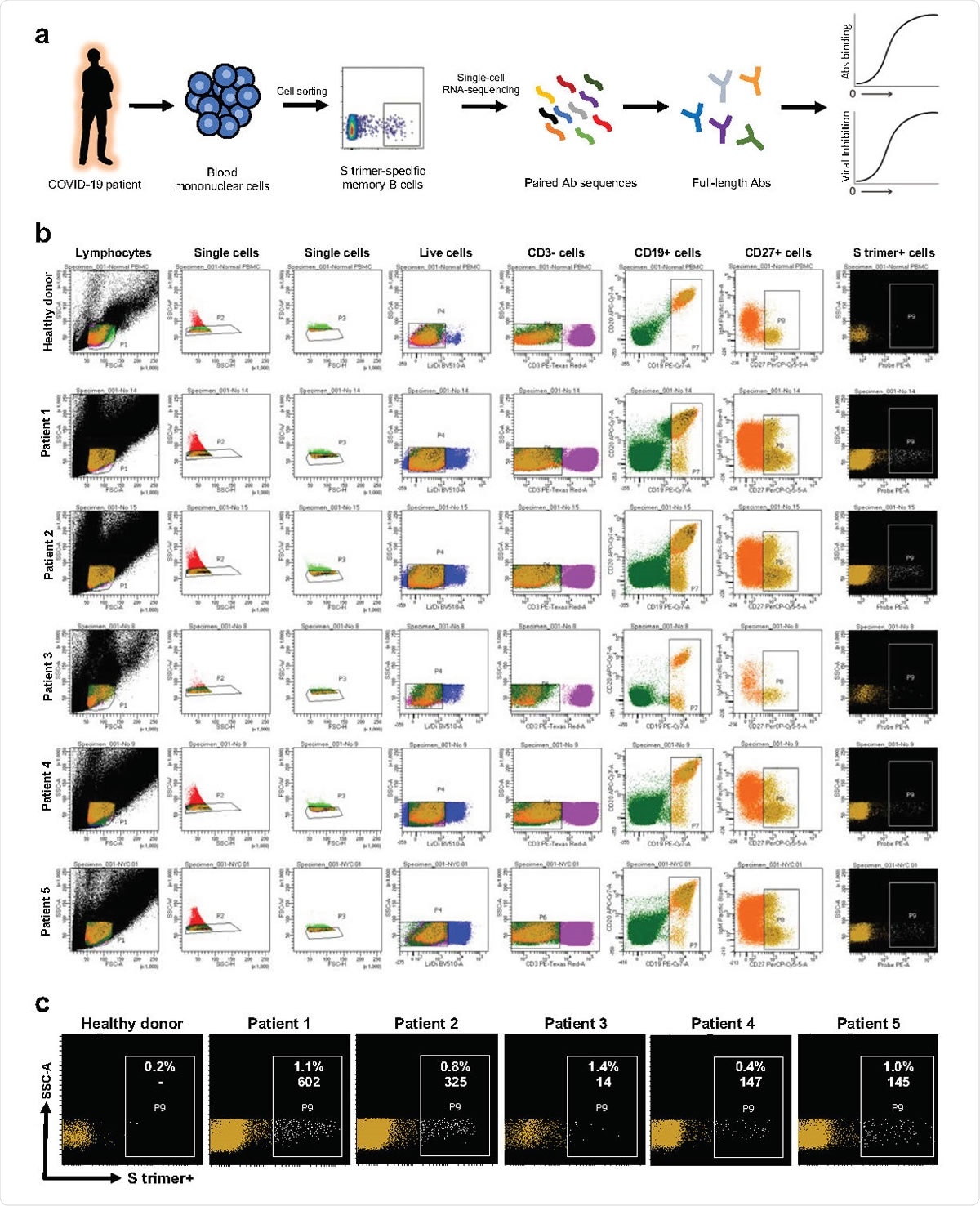
SARS-CoV-2 S trimer-specific antibody isolation strategy. a, Schema for isolating of S trimer-specific mAbs from memory B cells in the blood of infected patients. b, Sorting results on the isolation of S trimer-specific memory B cells using flow cytometry. c, Magnified representation of the panel of S trimer-positive memory B cells for each patient. Inset numbers indicate the absolute number and the percentage of S trimer-specific memory B cells isolated from each case.
What did the current study involve?
David Ho and colleagues recruited 40 infected patients, tested their blood plasma for virus-neutralizing antibodies, and selected five patients with the highest titers. These patients were all severely ill with respiratory disease that required mechanical ventilation.
“Previously, we observed that infected individuals with severe disease developed a more robust virus-neutralizing antibody response,” explains the team.
Enzyme-linked immunosorbent assays (ELISAs) were used to screen samples from the five patients for binding to the Spike protein and for neutralization against SARs-CoV-2.
Nine mAbs showed “exquisite potency”
Overall, 19 mAbs potently neutralized SARS-CoV-2, nine of which showed exquisite potency and inhibited 50% of the virus at concentrations of 9 ng/mL or less.
Four antibodies targeted the RBD; three targeted the NTD and two targeted nearby quaternary epitopes that overlapped with the two domains.
The researchers say the finding that non-RBD mAbs potently neutralized the virus is unprecedented since, until now, such antibodies have only been shown to exhibit much lower potencies. The finding that two mAbs targeted epitopes that do not map to RBD and NTD is also unprecedented, they add.
Potent neutralizing mAbs are directed towards the top of the Spike protein
According to the researchers, the approximately equal recognition of the RBD and NTD by mAbs indicates that both domains, which are located at the top of the Spike protein, are “quite immunogenic.”
Epitope mapping indicated that mAbs targeting the top of the RBD compete with ACE2 binding to neutralize the virus. In contrast, those targeting the side of this domain do not compete with ACE2 and have a weaker neutralizing effect.
Using Cryo-Electron Microscopy, the team provided first-time, high-resolution visualization of one mAb binding to the NTD and one binding to the RBD.
Furthermore, characterization of this diverse range of mAbs enabled the team to see that all-potent SARS-CoV-2-neutralizing antibodies so far described in the literature are directed towards the top of the Spike protein.
“Promising candidates” for SARs-CoV2 treatment
The team says that overall, these findings will help scientists understand how antibodies bind to and neutralize SARS-CoV-2.
“Surprisingly, many of these mAbs have V(D)J sequences close to germline sequences, without extensive somatic hypermutations, a finding that bodes well for vaccine development,” write the researchers.
“We believe several of our monoclonal antibodies with exquisite virus-neutralizing activity are promising candidates for development as modalities to treat or prevent SARS-CoV-2 infection,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources