The first study to elucidate the sequential dynamics of membrane cholesterol transport in erythrocytes infected with live Plasmodium falciparum parasites has been successfully concluded using the 3D label-free imaging capability of holotomography microscopy.
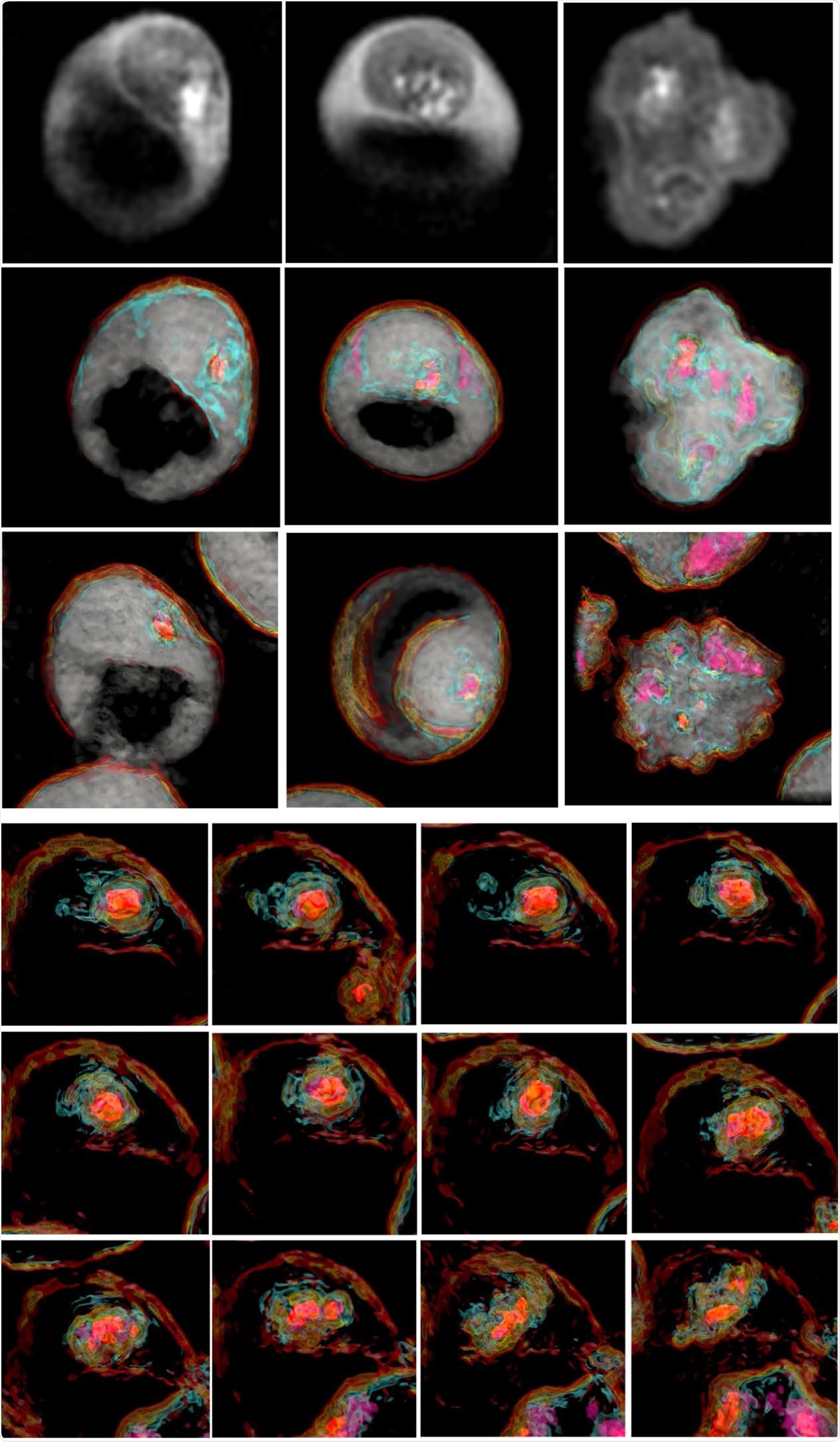
Image Credit: Tomocube
Writing in Nature Scientific Reports1, the international team of authors describes using the Tomocube HT-2H holotomographic microscope to observe in real-time the sequential membrane migration and alterations in the erythrocyte structure caused by the often-fatal parasite.
The observations allowed the joint Japanese-US researchers to suggest that a novel membrane transport system operates in the cytosol of P. falciparum-infected erythrocytes as a cholesterol import system, probably between the parasitophorous vacuole membrane (PVM) and the erythrocyte membrane, and that this transportation process occurs during the live erythrocyte stages of P. falciparum.
Despite a tremendous amount of research and the recent appearance of the first vaccine, malaria is still prevalent in tropical and subtropical regions of the world, particularly in Africa and East South Asia with an estimated 228 million cases in 2018 resulting in 405,000 deaths. The results of this latest study provide important insights to how P. falciparum, which lacks a cholesterol synthesis pathway, scavenges host erythrocyte cholesterol and might lead to new treatment pathways.”
Aubrey Lambert, Tomocube’s Chief Marketing Officer
Malaria, particularly when caused by P. falciparum, can be fatal within 24 hours of symptom onset with children aged under 5 years being the most vulnerable group accounting for two-thirds of all malaria deaths worldwide.
The control and eradication of the disease depend mainly on insecticide spraying and insecticide-treated bed nets to help to prevent the transmission of the infection via the mosquito vector. In addition, prevention and treatment through artemisinin-based combination therapy (ACT) is the WHO-recommended procedure for the majority of cases.
The Tomocube holotomography microscope delivers quantitative, nanoscale, real-time, 3-D images of individual living cells quickly and simply without any sample preparation. The holotomography images also deliver vital information on unique cell properties, including cell volume, shapes of sub-cellular organelles, cytoplasmic density, surface area, and deformability.
Although not used in the chromosome condensation study, the latest HT-2 model combines the quantitative phase imaging (QPI) approach of label-free, 3-D refractive index (RI) tomography with 3-D fluorescence imaging.
Winner of the Microscopy Today 2019 Innovation Award, this microscope enables long-term tracking of specific targets in live cells while minimizing stress. The capability to easily deliver holotomography and fluorescence correlative analysis in 2D, 3D and 4D will enable researchers and clinicians to open new frontiers in bioscience and better understand, diagnose, and treat disease.