In a breakthrough for research fields as diverse as diabetes, atherosclerosis and biofuels, the mass, concentration, volume, and surface area of individual lipid droplets have been measured in live, unstained cells for the first time using a Tomocube Holotomography (HT) microscope. The elimination of any preparative or staining steps, and the 110 nm lateral resolution of the Tomocube HT microscope, means that researchers can now easily track changes in lipid distribution over time in high resolution, enabling metabolic studies of cellular lipid content.
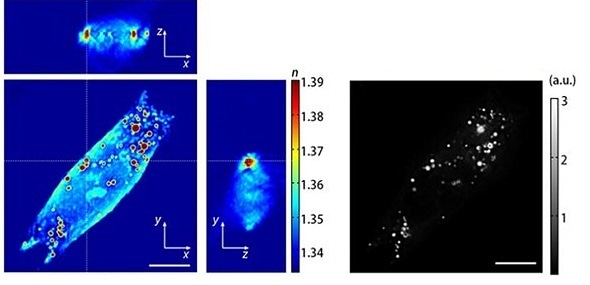
Holotomography of a human hepatocyte, Huh-7. 3D tomogram images (Left) fluorescence image (Right). High-RI droplets correlate to the lipid droplets stained by Nile red. 95.5% of organelles whose RI values are higher than n = 1.375 are overlapped to lipid droplets stained by Nile red. Reproduced from Kim et al. Scientific Reports 6:36815.
The immediate quantitative phase images of the intact morphology of live cells is a key advantage of HT microscopy compared to other imaging technologies. Holotomography detects the absolute refractive index (RI) of each voxel by measuring the phase delay. Since the RI of the lipid droplets is distinctly higher than the cytoplasm or other cellular components, the contrast is easily sufficient to image in high resolution. If the Tomocube HT-2H microscope is used, which supports both holotomography and 3D fluorescence imaging, co-localization experiments are possible through labelling just the target protein or specific type of fatty acid rather than having to stain both the lipid droplet and the target molecule.
Compare this to bright-field or phase contrast microscopy, which conveniently visualizes cellular morphology but only provide qualitative information. Confocal fluorescence microscopy does capture high-resolution 3D structural images but involves invasive labelling procedures that inevitably induce phototoxicity or photobleaching.”
Aubrey Lambert, Chief Marketing Officer, Tomocube
Lipid has a crucial role in modulating many cellular functions and a breakdown in its regulation is associated with diabetes, atherosclerosis and other metabolic diseases. The genetic engineering of lipid-rich bacterial and photosynthetic algae to increase the yield of fatty acids is also an important research area in the development of biofuels. In both cases, the amount of lipid needs to be quantified to study the metabolic dynamics in vitro.
Until now, visualizing the location of lipid required cells to be prepared using lipid-specific staining probes, such as Nile red or Oil red-O, interfering with the native cellular processes. Meanwhile, chromatography has been widely used to quantify lipid content although this only measures the average from hundreds of algae after time-consuming experimental procedures.
According to Lambert, “Holotomography is very suited to the detection of high RI molecules, which is a characteristic of lipid droplets. However, it is equally applicable to the study of other high-RI molecules, such as polymers, nanoparticles, or liposomes, and researchers can apply identical approaches to that of lipid imaging using HT microscopy.
“Rapid quantification of cellular lipid content is a unique feature of HT imaging and has huge potential for adoption in cell biology as well as in the fields of bioengineering or the biofuels industry.”