The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) circulating the world has caused over 9.6 million cases and over 490,000 deaths as of June 26, 2020. With neither an effective vaccine nor therapeutic drug yet available, intensive and widespread research is ongoing to develop a pharmacological intervention that will halt the global pandemic.
Now, a new study by an international team of researchers and published on the preprint server bioRxiv* in June 2020 reports that the drug pixatimod is a potent inhibitor of SARS-CoV-2 viral attachment and invasion in Vero cell culture, with a reduction of cytopathic effect when applied at concentrations within the established therapeutic concentrations.
COVID-19 disease, caused by SARS-CoV-2, is known to have three phases of the disease, that of early infection, the lung phase, and the severe hyperinflammatory phase. However, SARS-CoV-2 is not only a lung-tropic virus but also infects the gut cells and vascular endothelium. It can thus cause viral pneumonia with acute respiratory distress syndrome (ARDS) as well as cardiac and renal damage, and in the worst cases, multi-organ dysfunction.
Novel Coronavirus SARS-CoV-2 Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. Image at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Heparan Sulfate and Viral Entry
In searching for a molecule that can explain the viral tropism for multiple tissues, scientists have focused on heparan sulfate (HS). This is a sugar molecule present on most mammalian cells and serves as a receptor to many viruses. A variety of compounds that mimic the role of HS like heparin and other sulfur-carrying complex sugars are also known to inhibit the spread of viruses between cells and to block infectivity.
The SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) on the host cell to initiate virus entry. The S protein of the virus is responsible for this binding, and its S1 subunit carries a receptor-binding domain (RBD), which has an HS binding site. Both HS and heparin bind to the S1 subunit at the RBD, and trigger conformational changes in its structure. This led the researchers to think that the virus needs to bind to HS to enter the host cell. In this light, molecules that simulate the binding of HS could be effective as a therapy for this infection.
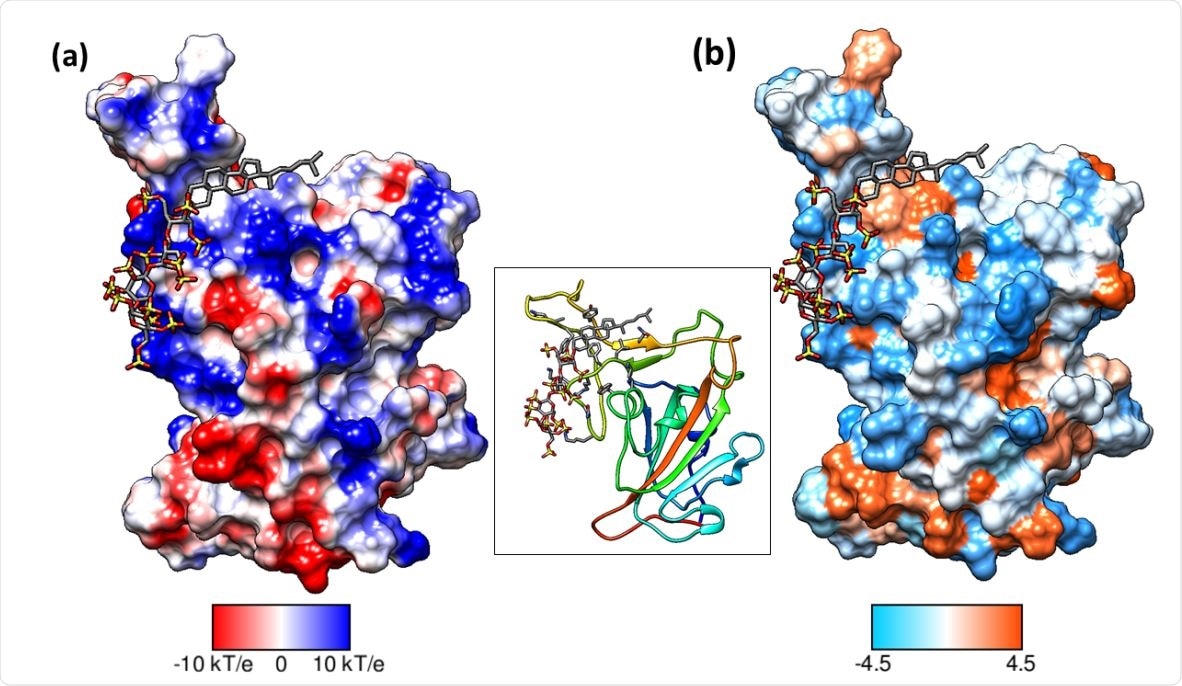
Binding mode of pixatimod on the S1 RBD (a) columbic surface and (b) hydrophobic surface. Both surfaces are oriented in the same direction as shown in the ribbon diagram of the protein in the middle. The sulfated tetrasaccharide interacts with the basic regions on S1 RBD whereas cholestanol residue prefers hydrophobic region for interactions. Coulombic surface coloring defaults: ε = 4r, thresholds ± 10 kcal/mol·e were used. Blue indicates surface with basic region whereas red indicates negatively charged surface. The hydrophobic surface was colored using the Kyte-Doolittle scale wherein blue, white, and orange-red color indicate most hydrophilicity, neutral and hydrophobic region, respectively. UCSF Chimera was used for creating surfaces and rendering the images. Hydrogens are not shown for clarity.
HS Mimetic Pixatimod
Pixatimod (PG545) is such a drug, which has entered the clinical stage of testing and has potential as an anticancer, anti-inflammatory, and antiviral drug against many viruses that use HS to enter the target cell. However, this molecule also has the unique ability to kill viruses by breaking up the lipid envelope, and thus prevent and treat viral infection with HSV-2/ Ross River virus, and dengue virus, respectively, as shown in several animal models.
Pixatimod has also been tested in some phase I trials for solid tumors and showed its safety and tolerability. The current study uses in vitro evidence of the interaction of pixatimod with the S1 protein RBD along with molecular modeling to support the clinical trial of the drug against SARS-CoV-2.
Pixatimod Binds Directly to the RBD
The molecular dynamics (MD) simulations were used to identify the binding sites of the drug on the S1 RBD surface. The scientists found 24 amino acid residues on the RBD that interacted with ACE2 residues, on X-ray crystal structures. They then found out which of these significantly interacted with pixatimod, and discovered that many of them were also involved in ACE2 binding.
Some RBD residues form transient hydrogen bonds or ionic bonds with the drug at its sulfated polysaccharide residue. Some residues also form interactions with the cholestanol residue to stabilize the structure.
The binding with pixatimod is energetically favored, more than the binding to an alternative site involving heparin, and it produced significant conformational changes at the N-terminal end of the RBD. This, therefore, robustly strengthens the theory that pixatimod binds directly to the RBD, perhaps bringing about a change in the conformation of the molecule. This change is different from that which occurs with the use of heparin, perhaps because both of them interact with the receptor in different ways. As a result, the drug could prevent RBD-ACE2 binding.
Pixatimod Inhibits Virus-Cell Binding
The researchers then carried out competitive ELISA tests, whereby the ability of the drug to displace biotinylated heparin that was already bound to immobilized viral RBD. The test showed that both heparin and pixatimod could displace the bound heparin, but only when present at high concentrations. Moreover, pixatimod displaced heparin less efficiently, which bears out the validity of the modeling data showing that their binding sites on the RBD overlap only in part. Heparin has greater avidity for binding because of its multiple binding sites.
The drug was then tested against the binding of the virus to the cells, to assess how well it could block the binding of RBD to Vero cells. The experiment showed a dose-response relationship with both heparin and pixatimod able to inhibit binding by about 50% and 30%, respectively. In other words, pixatimod can block the binding of the RBD to target cells bearing both ACE2 and heparan sulfate proteoglycans (HSPGs).
Pixatimod Reduces Cytopathic Effect and Plaque Formation
The next step was to carry out live virus assays with both pixatimod and the virus in Vero cell cultures. The number of plaque-forming units (PFUs) was significantly reduced when the virus was incubated with the drug before allowing cell infection. This drug was then assayed using a different isolate, and equivalent values were obtained. The efficacy was found to be high at values ranging from 0.8 to 13.8 μg/mL, which are well below the cytotoxic concentrations.
Using cytopathic effects as the endpoint, inhibition assays were carried out with the drug, showing equivalent efficacy as that observed in the plaque reduction assays. However, when the steroidal side-chain was removed, and the resulting molecule tested for plaque reduction, efficacy was lost, showing the side chain to be crucial in pixatimod efficacy.
Heparins and Pixatimod
Earlier studies show that heparin has multiple mechanisms whereby it may inhibit viral replication by binding to the RBD and that low molecular weight heparins (LMWHs) also share this ability to bind to and induce a conformational change in the RBD, though at a lower level since they were designed for anticoagulation.
LMWHs were also claimed to reduce IL-6 levels, which is vital in preventing the severe phenotype of COVID-19. However, these drugs may not be suitable for treating this infection, and some scientists recommend their use only in patients with confirmed thrombosis, to avoid the ever-present risk of serious hemorrhage.
HS has the advantage of not causing bleeding and could be used to prevent and treat COVID-19. Pixatimod could thus offer better opportunities for structural design, molecular weight regulation, sulfation, as well as for attributes like purity and stability, to create an optimal pharmaceutical product. The low anticoagulant activity is another advantage allowing it to be used in conjunction with heparin and LMWHs (which are used to counter thrombotic tendencies) without the danger of inducing bleeds.
Pixatimod: Inhibition and Immunomodulation
Finally, pixatimod may have an immunomodulatory activity such as inhibiting heparinase and IL-6, both of which are pro-inflammatory triggers. It could also, via heparanase inhibition, block macrophage invasion of monocytes and macrophages into infected lung tissue, as seen in severe COVID-19, judging from the macrophage invasion seen in tumors in animal models. Thirdly, heparinase is known to be responsible for disease progression in viral infection by breaking down HS and thus allow the virus to spread across epithelial barriers to distant sites.
The researchers sum up: “Pixatimod effectively inhibits viral infection of Vero cells. Importantly, its potency is well within its safe therapeutic dose range. This provides a strong rationale for its clinical investigation as a new multimodal therapeutic for the current COVID-19 pandemic.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Guimond, S. E. et al. (2020). Pixatimod (PG545), A Clinical-Stage Heparan Sulfate Mimetic, Is A Potent Inhibitor of the SARS-CoV-2 Virus. bioRxiv preprint. doi: https://doi.org/10.1101/2020.06.24.169334. https://www.biorxiv.org/content/10.1101/2020.06.24.169334v1
- Peer reviewed and published scientific report.
Guimond, Scott E., Courtney J. Mycroft-West, Neha S. Gandhi, Julia A. Tree, Thuy T. Le, C. Mirella Spalluto, Maria V. Humbert, et al. 2022. “Synthetic Heparan Sulfate Mimetic Pixatimod (PG545) Potently Inhibits SARS-CoV-2 by Disrupting the Spike–ACE2 Interaction.” ACS Central Science 8 (5): 527–45. https://doi.org/10.1021/acscentsci.1c01293. https://pubs.acs.org/doi/10.1021/acscentsci.1c01293.