A diverse research group from the United Kingdom, Italy, China, and Australia established a first expandable human gastric organoid culture across fetal developmental stages, supporting the hypothesis that fetal tissue seems to be much less susceptible to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection – especially in the early stages of development. The findings are described in the paper currently available on the bioRxiv* preprint server.
.jpg)
Novel Coronavirus SARS-CoV-2 Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
When coronavirus disease (COVID-19) pandemic is concerned, it is still not completely clear how gastrointestinal viral replication might subsequently affect the clinical outcome of infection, transmission dynamics in the population, or the development of protective immune response.
And while it has been demonstrated that SARS-CoV-2 is habitually found in rectal samples of affected children and adults, it remains to be seen whether the virus can induce a primary infection throughout the entire gastrointestinal tract, or if it is just partly related to a passive transport of contaminated sputum that stems from the upper respiratory tract.
Most importantly, the propensity of SARS-CoV-2 to persist in the gastrointestinal tract after full respiratory clearance has not yet been elucidated in terms of viral infectivity, possibly hampering essential public health and policy measures for the control of the disease.
These concerns are particularly relevant in children who usually suffer a much less severe respiratory illness when compared to adults, regardless of more prominent gastrointestinal symptoms that can mimic appendicitis or a hyperinflammatory shock syndrome.
A research group led by Dr. Giovanni Giuseeppe Giobbe from the University College London and Dr. Francesco Bonfante, the Istituto Zooprofilattico Sperimentale delle Venezie in Italy, aimed to unravel the susceptibility of the stomach to SARS-CoV-2 infection by developing a cutting-edge expandable laboratory model that accurately reproduces the gastric microenvironment.
Priming and infecting gastric-derived organoids
In order to derive a completely novel in vitro gastric model of fetal origin, the researchers initially characterized the human fetal tissues and subsequently compared them to gastric mucosa obtained from pediatric patients undergoing surgery.
Then they have defined three specific groups of gastric epithelial tissue based on gland maturity: early fetal stomachs between post-conception weeks 8-15, late fetal stomachs between post-conception weeks 17-21, and true pediatric stomachs.
After thorough gastric tissue characterization, this research group extracted glandular crypts from fetal and pediatric stomachs by using mechanical stress and chelating buffers. The transcriptomics approaches were employed to characterize gastric epithelial tissues and gastric-derived organoids at three developmental stages.
In order to validate aforementioned organoids as functional laboratory models of SARS-CoV-2 infection and replication, the cultures were optimized for viral infection in a three-dimensional system. A reversed polarity in the gastric organoids was induced to appraise SARS-CoV-2 infection with minimal perturbation and under steady-state conditions.
Moreover, RNA sequencing (i.e., a technique that employs next-generation sequencing to ascertain the presence and quantity of RNA in a certain biological sample) on both non-infected and infected organoids was pursued at each developmental stage, as well as an enrichment analysis within the open-source Reactome pathway database.
Replication of SARS-CoV-2 scarce in fetuses, but efficient in children
"Collectively, we established the first expandable human gastric organoid culture across fetal developmental stages, and we support the hypothesis that fetal tissue seems to be less susceptible to SARS-CoV-2 infection, especially in early stages of development", study authors summarize their findings.
On the other hand, they have also shown that SARS-CoV-2 can efficiently infect gastric epithelium in pediatric patients, which implies that the stomach might be an active player in the fecal-oral transmission of this putative viral agent. Actually, the replication of SARS-CoV-2 is increasing across the developmental stages of the gastric organoids.
The susceptibility of pediatric gastric organoids is evidenced by the expression of viral nucleoprotein in cells undergoing programmed cell death, which is much less the case in fetal organoids – suggesting, in turn, lower efficiency of infection.
Furthermore, the transcriptomic analysis revealed a moderate innate antiviral response, but also the lack of differentially expressed genes which belong to the interferon family. The expression of gastric markers (such as gastrin, somatostatin, pepsinogen, and chromogranin A) was more pronounced in pediatric organoids when compared to the fetal ones.
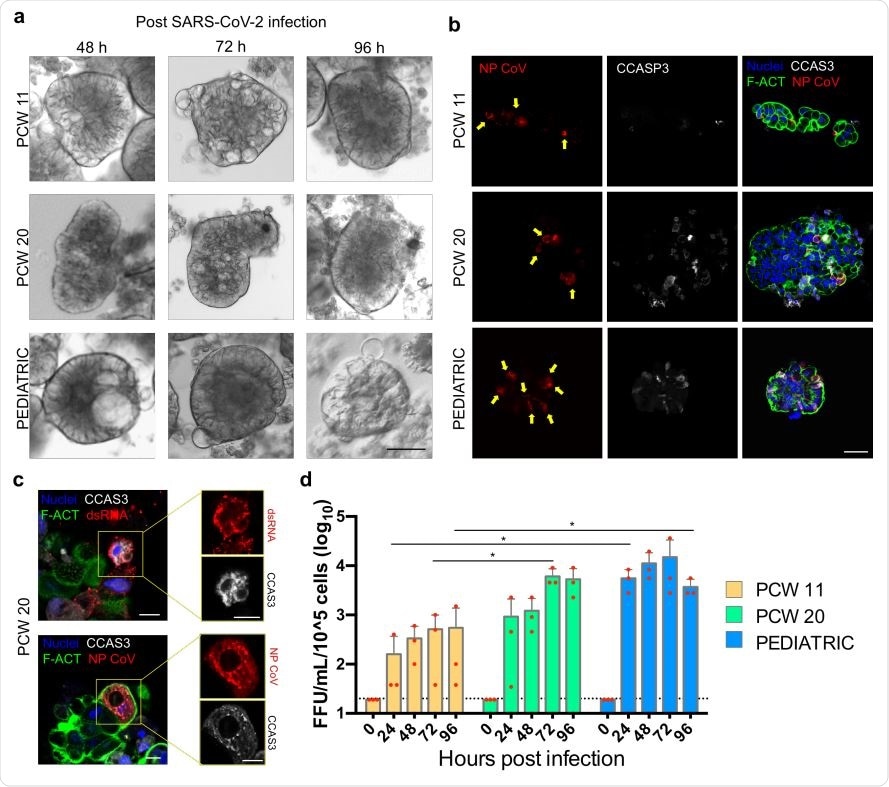
SARS-CoV-2 infection of human fetal and pediatric gastric organoids. a) Bright-field images of reversed-polarity organoids infected with pediatric patient-derived SARS-CoV-2 for 2 hours, and acquired at 48, 72 and 96 h post-infection. b) Infected cells in reversed organoids fixed at 96 h post-infection. Immunofluorescence panel showing SARS-CoV-2 Nucleocapsid Antibody (NP CoV) in red, marking infected cells (yellow arrows), cleaved caspase 3 (CCAS3) in white, marking apoptotic cells, f-actin (F-ACT) in green and nuclei in blue (Hoechst). The image is representative of at least 5 similar organoid images. Scale bar 50 µm. c) Infected cell details in reversed organoids fixed at 96 h post-infection. Immunofluorescence panel showing viral double-strand RNA J2 (dsRNA) in red, SARS-CoV-2 Nucleocapsid Antibody (NP CoV) in red, cleaved caspase 3 (CCAS3) in white, f-actin (F-ACT) in green and nuclei in blue (Hoechst). Scale bars 10 µm. d) Graph of SARS-CoV-2 replication in fetal and pediatric reverse-polarity organoids. Live virus yield of reverse-polarity organoids was titrated by FFA on Vero E6 cells of culture supernatants collected at 0, 24, 48, 72, and 96 hours after infection with SARS-CoV-2. The dotted line indicates the lower limit of detection. Red dots indicate single data points. Mean ± SD (N=3).
A scalable laboratory platform for drug research
"Our gastric organoid system offers a unique tool to characterize the replication of viruses and some of the associated pathological consequences of infection," study authors explain the significance of their research endeavor outlined in bioRxiv paper.
In addition, due to a deeper understanding of pathogenic mechanisms underlining viral colonization of the gastrointestinal system, this innovative model could represent a scalable laboratory platform for developing and testing antiviral drug candidates against SARS-CoV-2 to suppress viral shedding and halt the spread of the disease.
"The clinical importance of our findings relates to the worrisome phenomenon of prolonged shedding of SARS-CoV-2 from the gastrointestinal tract and calls for further research to assess the risk of vertical transmission in infected women", conclude study authors.
In any case, precisely defining anatomical target sites and the susceptible age range will be of utmost importance if we are to implement more sensitive and sustainable diagnostic screening approaches to find contagious asymptomatic patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Giobbe, G.G., Bonfante F., et al. (2020). SARS-CoV-2 infection and replication in human fetal and pediatric gastric organoids. bioRxiv. https://doi.org/10.1101/2020.06.24.167049.
- Peer reviewed and published scientific report.
Giobbe, Giovanni Giuseppe, Francesco Bonfante, Brendan C. Jones, Onelia Gagliano, Camilla Luni, Elisa Zambaiti, Silvia Perin, et al. 2021. “SARS-CoV-2 Infection and Replication in Human Gastric Organoids.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-26762-2. https://www.nature.com/articles/s41467-021-26762-2.