Researchers in the U.S. have shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may alter key protein structures on red blood cells and compromise the transport and delivery of oxygen in patients with coronavirus disease 2019 (COVID-19).
Altered protein-membrane homeostasis may contribute to clot formation and the coagulation complications that are sometimes observed in severely ill patients, say Steven Spitalnik (Columbia University, New York) and colleagues.
A pre-print version of the paper is available in the server medRxiv*, while the article undergoes peer review.
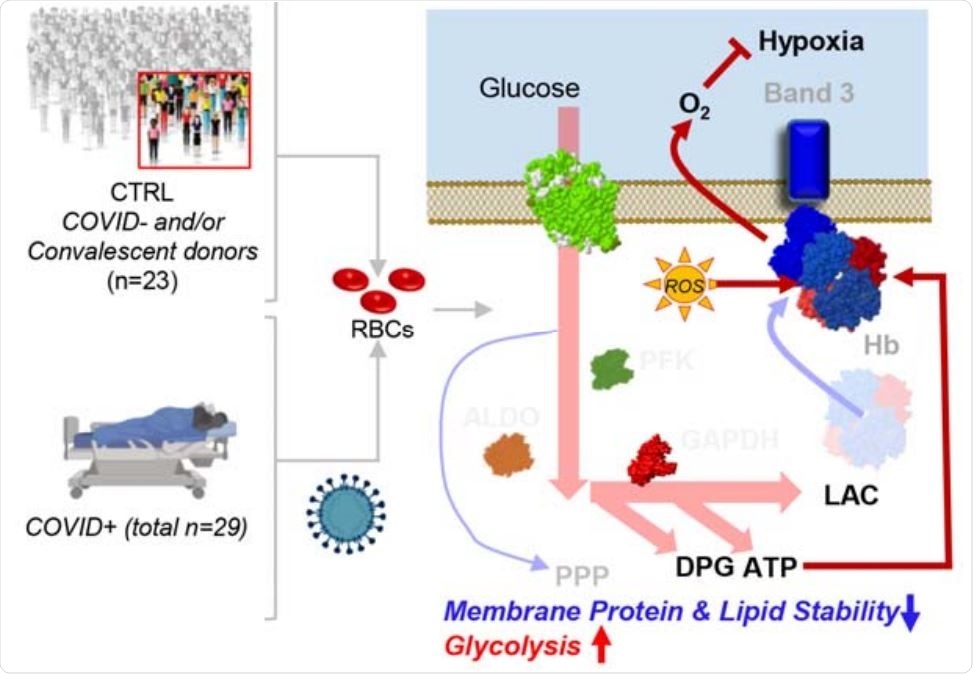
Susceptibility of RBCs to SARS-CoV-2

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
COVID-19 is primarily characterized by a dry cough, shortness of breath, and in severe cases, respiratory failure and death.
The causative agent – SARS-CoV-2 – infects cells by using its Spike protein to bind host angiotensin-converting enzyme 2 (ACE2) and fuse with the cell membrane. This ACE2 receptor is abundantly expressed on lung alveolar epithelial cells.
According to Spitalnik and colleagues, proteomics studies have previously identified angiotensin and ACE2-interacting proteins on the surface of RBCs, suggesting that these cells may be susceptible to SARS-CoV-2 invasion.
Since RBCs are responsible for transporting oxygen around the body, their alteration may contribute to the severity of hypoxemia among patients with severe COVID19, suggests the team.
“Because RBCs are critical for oxygen transport and off-loading, the severely low oxygen saturations seen in critically ill COVID-19 patients suggest the importance of determining whether SARS-CoV-2 infection directly or indirectly affects RBC metabolism to influence their gas transport, structural integrity, and circulation in the bloodstream,” they write.
Comparing RBCs between COVID-19 patients and healthy controls
Now, the researchers have used state-of-the-art metabolomics, proteomics, and lipidomics techniques to explore the effect of COVID-19 on RBCs. They compared blood samples taken from 29 patients confirmed as SARS-CoV-2 positive by molecular testing of nasopharyngeal swabs with samples from 23 controls who were molecularly negative for infection by nasopharyngeal swabs.
“The present study provides the first comprehensive multi-omics analysis of RBCs from non-infected control and COVID-19 patients, identified by molecular testing of nasopharyngeal swabs,” say Spitalnik and colleagues.
The team reports that compared with controls, RBCs from COVID-19 patients exhibited increased glycolysis, with significantly increased sucrose consumption and accumulation of glycolytic intermediates such as glucose 6-phosphate, phosphoglycerate, pyruvate, lactate, and NADH.
This increased glycolysis was accompanied by increased oxygenation and fragmentation of essential membrane proteins, including ankyrin, spectrin beta, and the N-terminal of anion exchanger 1 (AE1).
RBCs from COVID-19 patients also had significantly altered lipid metabolism, with lower levels of acyl-carnitines and short-chain fatty acids and higher levels of long-chain saturated fatty acids.
These alterations to RBCs in COVID-19 patients were not accompanied by any changes in clinical hematological parameters such as RBC count and hematocrit and only minor increases in mean corpuscular volume.
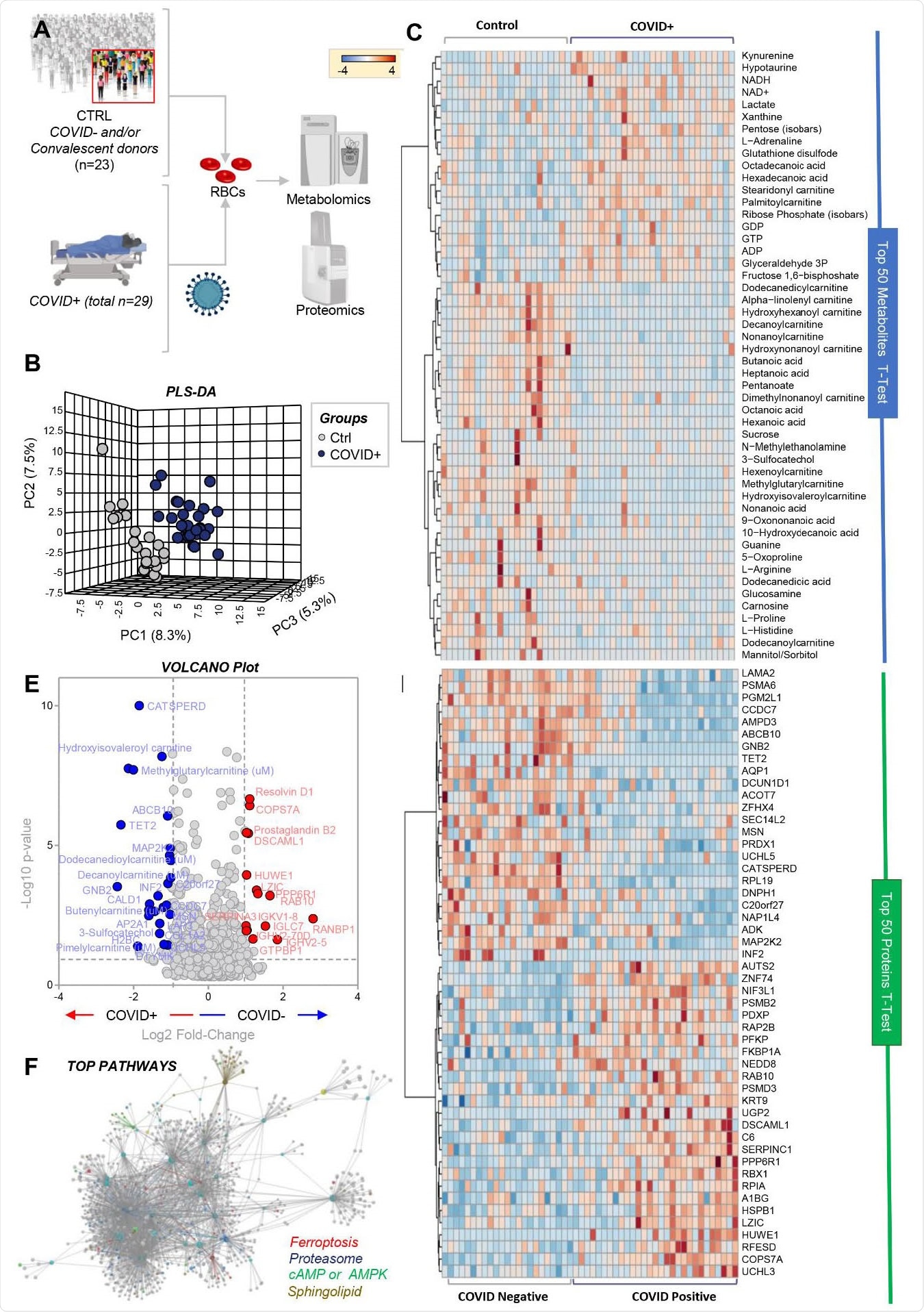
RBC metabolism and proteome are influenced by COVID-19. Metabolomics and proteomics analyses were performed on RBCs from COVID-19-negative (n=23) and -positive (n=29) subjects, as determined by molecular testing of nasopharyngeal swabs (A). The effects of COVID-19 on RBCs, as gleaned by PLS-DA (B) and hierarchical clustering analysis of the top 50 metabolites (C) and proteins (D) by t-test. In E, the volcano plot highlights the significant metabolites and proteins increasing (red) or decreasing (blue) in RBCs from COVID-19 patients, as compared to non-infected controls. In F, pathway analyses were performed on the significant features from the analyses in B-E.
“These results suggest a significant impact of SARS-CoV-2 infection on RBC”
“Taken together, these results suggest a significant impact of SARS-CoV-2 infection on RBC structural membrane homeostasis at the protein and lipid levels,” say Spitalnik and colleagues.
Theoretically, increases in RBC glycolytic metabolites could increase the capacity of hemoglobin to off-load oxygen as a function of modulation by high-energy phosphate compounds, possibly to counteract COVID-19-induced hypoxia, suggest the researchers.
However, “because the N-terminus of AE1 stabilizes deoxyhemoglobin and finely tunes oxygen off-loading, RBCs from COVID-19 patients may be incapable of responding to environmental variations in hemoglobin oxygen saturation when traveling from the lungs to peripheral capillaries,” they add. This would then compromise the capacity of RBCs to transport and deliver oxygen around the body.
Spitalnik and the team said they could not directly measure these parameters related to gas transport due to logistical limitations, but that this possibility will be addressed in follow-up studies.
The researchers also say the increased oxidation of key structural proteins and altered lipid metabolism may change the morphology and deformability of RBCs since these parameters are usually tightly regulated by structural protein and lipid homeostasis.
“As such, the altered RBC structural proteins in COVID-19 may contribute to the thromboembolic and coagulopathic complications seen in some critically ill patients,” they write.
However, more extensive studies will be needed to test this hypothesis, concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources