The coronavirus pandemic has spread across the globe, infecting more than 10 million people. As it continues to wreak havoc, many scientists are working to determine a potential weakness in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) so as to find an effective drug or vaccine against it.
A team of scientists at the University of California San Francisco has found out how SARS-CoV-2, the virus that causes the coronavirus disease (COVID-19) invades proteins in the cells that serve as master regulators of key cellular processes. The virus can rewire the cell’s circuits to promote its survival and proliferation. The scientists also suggest that targeting the pathogen’s reliance on host-cell proteins could be its Achilles’ heel or weakness.
The study, published in the journal Cell, highlights the possibility of targeting the virus’s weakness so it could be killed, potentially stopping the global pandemic.
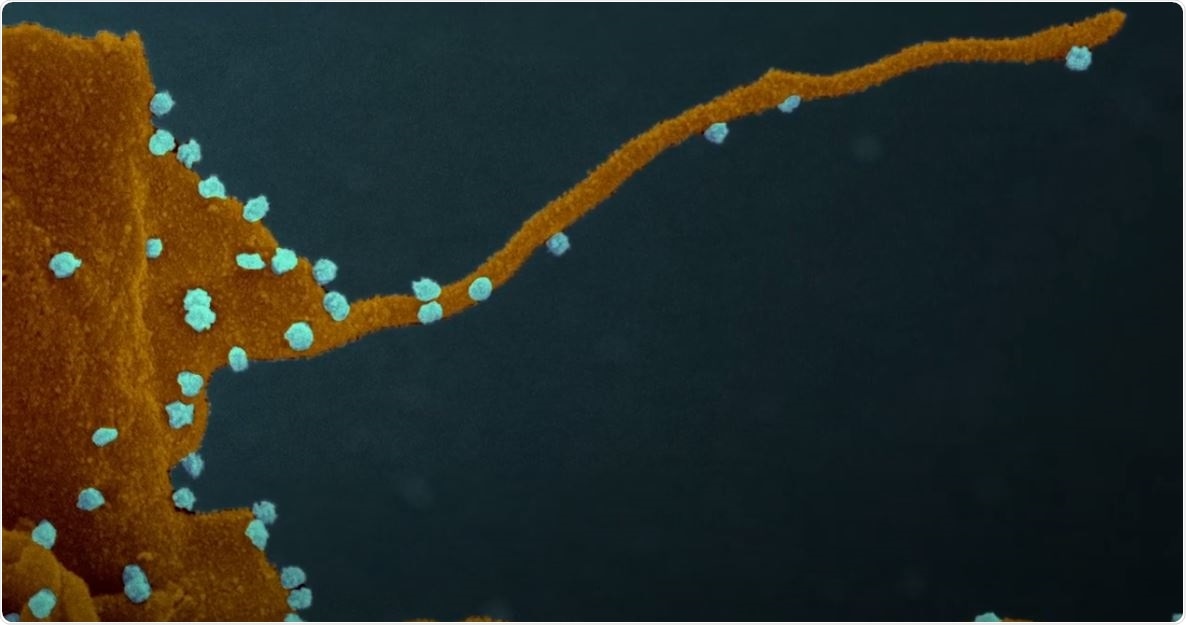
Coronavirus forces cells to produce tentacle-like structures that infect neighboring cells
How does SARS-CoV-2 infect cells?
The SARS-CoV-2 is a rapidly spreading virus that has ravaged across the globe, killing more than 515,000 people. A key to stopping the spread of the virus is to understand how it enters the cells.
SARS-CoV-2 uses the human angiotensin-converting enzyme 2 (ACE2) as an entry receptor to gain access to the cells. The virus’s spike proteins bind to the ACE2, which plays a role in regulating blood pressure. When the virus binds to it, it sets off chemical changes that can fuse the membranes around the cell and virus together. In turn, the RNA of the virus can enter the cell.
From there, the virus hijacks the cell’s protein-making machinery to translate its RNA into new copies of the virus. Within a few hours, a single cell can produce many new virions, which can infect other cells in the body.
Key cell entry mechanism
The team has identified key cell entry mechanisms of SARS-CoV-2 that can potentially contribute to the immune evasion, cell infectivity, and the wide spread of the virus.
They also found that when SARS-CoV-2 infects cells, it gets in control over a group of enzymes called kinases. Usually, kinases play a pivotal role as master regulators of growth, metabolism, repair, movement, and other vital cellular processes. They attach small chemical tags to proteins via a process known as phosphorylation. After attachment, the tags act as switches that can turn proteins on or off, allowing the complex machinery to work correctly and smoothly.
Coronavirus forces cells to produce tentacle-like structures that infect neighboring cells
However, when the SARS-CoV-2 gains control of the cell, the kinases may behave in a different way, which may alter cell function and transform the host cell into a virus factory. These possessed cells then develop streaming filaments or filopodia, which are tentacle-like structures that bore into the cells’ bodies and inject their viral venom into the genetic command centers of the cells, creating another virus factory.
The researchers noted that these new dendrites strengthen the efficiency of SARS-CoV-2 in capturing new cells and establishing infection in humans.
Even though other viruses, including Marburg, Ebola, and vaccinia, are known to create filopodia, this is the first time that they have been observed in coronaviruses. The scientists also believe that the novel coronavirus may use filipodia as an infective transport system.
Potential drugs
The scientists also believe that they have identified several drugs that could help alter the viral takeover of cells. These drugs may have the potential to treat patients who are infected with the coronavirus.
“Eighty-seven drugs and compounds were identified by mapping global phosphorylation profiles to dysregulated kinases and pathways. We found pharmacologic inhibition of p38, CK2, CDKs, AXL, and PIKFYVE kinases to possess antiviral efficacy, representing potential COVID-19 therapies,” the researchers wrote in the paper.
Among the drugs identified include cancer drugs that work by blocking the chemical signals that activate the production of filipodia. These include Silmitasertib, an experimental drug for treating bile duct cancer, Ralimetinib, a small molecule experimental cancer drug in development by Eli Lilly.
“We are encouraged by our findings that drugs targeting differentially phosphorylated proteins inhibited SARS-CoV-2 infection in cell culture. We expect to build upon this work by testing many other kinase inhibitors while concurrently conducting experiments with other technologies to identify underlying pathways and additional potential therapeutics that may intervene in COVID-19 effectively,” Dr. Kevan Shokat, a professor of cellular and molecular pharmacology at UCSF and co-senior author of the study, said.
Sources:
Journal reference:
- Bouhaddou, M., Memon, D., Meyer, B., Swaney, D., Beltrao, P., Krogan, N. et al. (2020). The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell. https://www.cell.com/cell/fulltext/S0092-8674(20)30811-4