The COVID-19 pandemic is continuing to cause tens of thousands of infections daily. Without either an antiviral preventive, therapeutic, or vaccine, scientists are working at a hectic pace to find a solution. Now, a new study published on the preprint server bioRxiv* in June 2020 shows that a candidate spike protein vaccine can induce neutralizing antibodies, antiviral T cell responses, and protection against infection.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is studded with spikes composed of glycoprotein. These are necessary for receptor binding, which triggers viral endocytosis followed by membrane fusion between the host cell and the virus. This allows the virus to enter the cytoplasm and replicate. It also stimulates the host immune response.
The spike glycoprotein is a fusion protein, an inactive precursor. At the S1/S2 interface, the protein has a uniquely inserted polybasic furin cleavage site, not seen in the earlier SARS-CoV. The binding of the S protein, at the receptor-binding domain (RBD), to the host cell receptor, causes cleavage of the protein at the interface, generating the S2 subunit, which is conserved across all the coronaviruses found in human infections, and the S1 cap which is more variable.
In addition to the RBD, the S1 subunit also carries the N-terminal domain, while the S2 subunit carries the fusion peptide and other domains. The S protein occurs as a non-covalently associated trimeric protein. Like other fusion proteins, the trimeric S protein undergoes a conformational change from its prefusion to the postfusion conformation, following its binding to the host cell receptor and proteolytic cleavage.
The effect of this structural change is the exposure of the hydrophobic fusion peptide, which allows the virus to insert into the host cell membrane. Subsequent steps include the alignment of the virus-host membrane, their fusion, and endocytosis of the virus, whereby it enters the cytoplasm.
The Study: Immunizing Animals with Spike-Based Vaccine
The current study reports the development of a spike subunit vaccine based on the full-length spike protein of SARS-CoV-2. The stable prefusion S protein structure used in this vaccine was obtained by introducing a mutation at the furin cleavage site, whereby two proline residues were substituted at the center, making this site resistant to cleavage. It shows high-affinity binding to the human ACE2 receptor.
The selected strain, labeled NVX-CoV2373, was thermostable, showed specific and high-affinity binding to the receptor, and was stable under stressed conditions such as prolonged agitation, freeze-thaw cycles, and high temperatures.
The study utilized both mice and nonhuman primates (NHP) to test the vaccine strain for antigenicity and protection. This results in blocking antibodies against the human ACE2 receptor and neutralizing antibodies against SARS-CoV-2. The vaccine was coupled with a saponin-based Matrix-M adjuvant to test the effect on immunogenicity.
High Antibody Titers
Mice were immunized with one priming dose or with one priming and one booster, 14 days apart, at any of four low doses (0.01 μg, 0.1 μg, 1 μg, and 10 μg) with 5 μg Matrix-M adjuvant.
Mice who received one dose at any of these dosages developed high anti-S IgG titers at 3-4 weeks after immunization. Those that received 10 μg also developed blocking and neutralizing antibodies within this period with a single priming dose.
When a booster dose was added, higher IgG antibodies were present 7-16 days from the booster. Mice immunized with 1 μg or 10 μg of the adjuvanted vaccine developed similar high antibody titers. At all dosages, the prime/boost schedule with adjuvanted vaccine elicited high levels of blocking and neutralizing antibodies to the S protein.
The use of an adjuvant significantly enhanced the antibody titer – at doses of 0.1 μg, 1 μg, or 10 μg, the mice who received the adjuvanted vaccine had higher titers than at the highest dose without adjuvant.
Higher B and T Cell Frequencies
The development of functional immunity depends on both components, the viral antigen, and the adjuvant. The frequency of IFN-γ+, TNF-α+, and IL-2+ cytokine-secreting CD4+ and CD8+ T cells was much higher in animals who received the adjuvanted vaccine compared to the plain vaccine.
They also showed a higher frequency of multifunctional CD3 and CD8 T cells – T cells that secrete multiple cytokines – in those who received the adjuvanted vaccine. The resulting CD4 T cell development was Th1 dominant or at least balanced between Th1 and other T cell phenotypes.
The immunization also induced both T follicular helper (Tfh) cells and germinal center B cell development in the spleen, which are essential to maintain the production of high-affinity antibodies. They found that when coupled with adjuvant, the vaccine-induced a more robust immune response in both groups of mammals, with the dominant role being played by T helper (Th) 1 cells, involving both B and T cells of CD4 and CD8 subtypes
The researchers comment, “These results indicate the potential for a 10-fold or greater dose sparing provided by Matrix-M adjuvant.”
Protection Against Infection
In mice infected with an adenovirus vector expressing the human ACE2 receptor so that they could support the replication of the SARS-CoV-2 strain, the results were striking. The vaccine prevented the development of clinical infection, without the vaccine-associated enhanced respiratory disease (VAERD).
Whereas unimmunized mice had 10,000 plaque-forming units (PFU) of the virus per lung, those immunized with the vaccine alone, without adjuvant, had 1,000 copies per lung, and those who received the adjuvanted vaccine had no detectable viral load.
In summary, at higher doses, mice immunized with a single priming dose showed no detectable virus. When a booster dose was also administered, all dosages resulted in protection against infection, or at least 1 log reduction in load compared to unimmunized mice. The same trends were mirrored concerning weight loss in immunized vs. non-immunized animals.
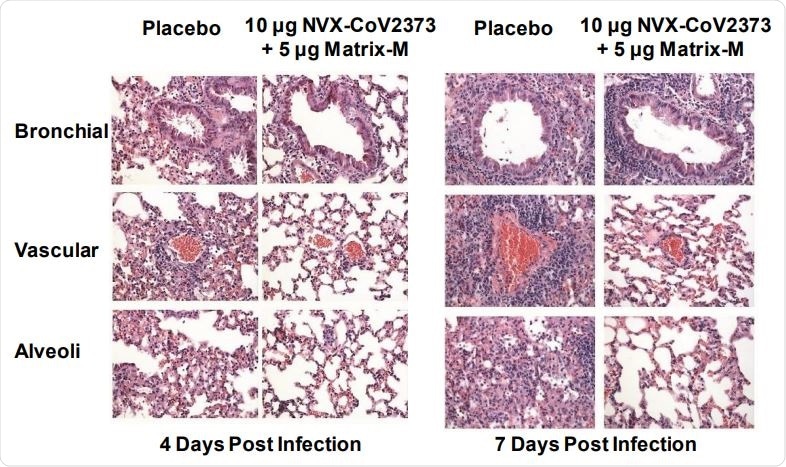
Representative histopathology of lungs from NVX-CoV2373 vaccinated 773 and Ad/CMV/hACE2 transduced mice challenged with SARS-CoV-2.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Nonhuman Primate and COVID-19 Patient Sera
In baboons, the vaccine-induced anti-S IgG within 21 days of a single priming dose at all dosages (1 μg, 5 μg, and 25 μg), while the antibody titers increased by one log or more within two weeks from the booster, again at all dosages. When plain vaccine without adjuvant was used, the antibody was minimal or undetectable after the first and second doses.
Receptor blocking antibody levels were low after the first dose at all dosages but increased significantly after 1-2 weeks of the booster. Neutralizing antibodies were also increased at all dosages after one dose and went up by 25-38 fold after the second dose. However, the plain vaccine did not induce blocking or neutralizing antibodies.
When compared with the antibody levels in recovered COVID-19 patients, the titers were seven times higher in the baboons than in the latter, along with 8-fold higher receptor binding and blocking antibodies.
Implications
These findings indicate the highly promising nature of this candidate for further development as a clinical vaccine. It is now undergoing phase 1 and 2 clinical trials for evaluation of its safety and immunogenicity in humans.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources