With the increasingly detailed exploration of the structure and function of the RNA genome of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that is causing the current COVID-19 pandemic, new light is being shed on many functional aspects of the virus. Now, a new Swiss study published on the preprint server bioRxiv* in July 2020 shows that the 5’ end of the RNA molecule is capable of inhibiting the binding of mRNA to the ribosomal 40S subunit, thus preventing translation.
Once the virus enters a host cell, the genomic RNA is translated by the cellular machinery to produce non-structural proteins (nsps). These are required to allow viral infection and viral mRNA synthesis.
Coronaviruses are observed to have adapted to their hosts by a slew of mechanisms to achieve these ends. Some of these are inhibition of protein synthesis by the host cell, as well as splitting mRNAs produced by the host cell. In the SARS-CoV, the host shutoff protein has been observed, which is a confounding factor called nsp1. Encoded by the first gene at the 5’ end, this is expressed first of all and is intended to close down the expression of host proteins, including immune proteins, immediately after the host cell is entered and infected.
The examination of this molecule shows that while the N-terminal domain is structured, the C-terminal domain is flexibly disordered. By acting against the expression of type I interferon and antiviral molecules, nsp1 is a factor that possibly increases the virulence of the virus, and could be the basis for a live attenuated vaccine.
The Study: How Translation Inhibition Occurs
The current study aims to understand how nsp1 inhibits translation at the molecular level, how this can be suppressed by key mutations, and how it enhances the translation of reporters that contain full-length UTRs of the virus. This may explain how the nsp1 can suppress host translation but allow viral mRNAs to be translated.
The researchers found that when the bacterially produced viral nsp1 was added to ribosomal particles, they found that it binds to both 40S and 80S complexes. CryoEM of the pooled ribosomal complexes showed that a preinitiation complex (PIC) was formed comprising several proteins, including the initiation factor elF3 core and initiator tRNA. Surprisingly, there was a density in the entrance channel of the mRNA, which was not due to the mRNA.
They found that nsp1 binds only to 40S ribosomal subunits. This complex was therefore assembled and its structure examined by cryoEM. This revealed that the density was the C-terminal domain of nsp1.
Docking analysis of the resulting molecular model into the 43S molecular map obtained earlier showed that the C-terminal end of this nsp1 is also found in association with the PIC. At high resolution, it was apparent that the C-terminal portion was within the mRNA entrance channel. This agrees with the findings in the 40S-nsp1 complex.
The nsp1 protein folded at the C-terminal part to form a pair of helices that engaged to the h18 of the 18S mRNA and to proteins uS3 of the head, and to uS5 and eS30, in the body, respectively. These form a network of interactions through multiple side chains.
With both complexes, nsp1 binds to the mRNA entrance channel present in the 40S subunit. Here it overlaps with the mRNA even if the latter is wholly accommodated, leading to the inhibition of mRNA by nsp1 binding.
The researchers say, “Nsp1 is tightly bound to the 40S subunit through anchoring of its C-terminal helices to the mRNA channel while the N-terminal domain can sample space in the radius of approximately 60 Å from its attachment point.”
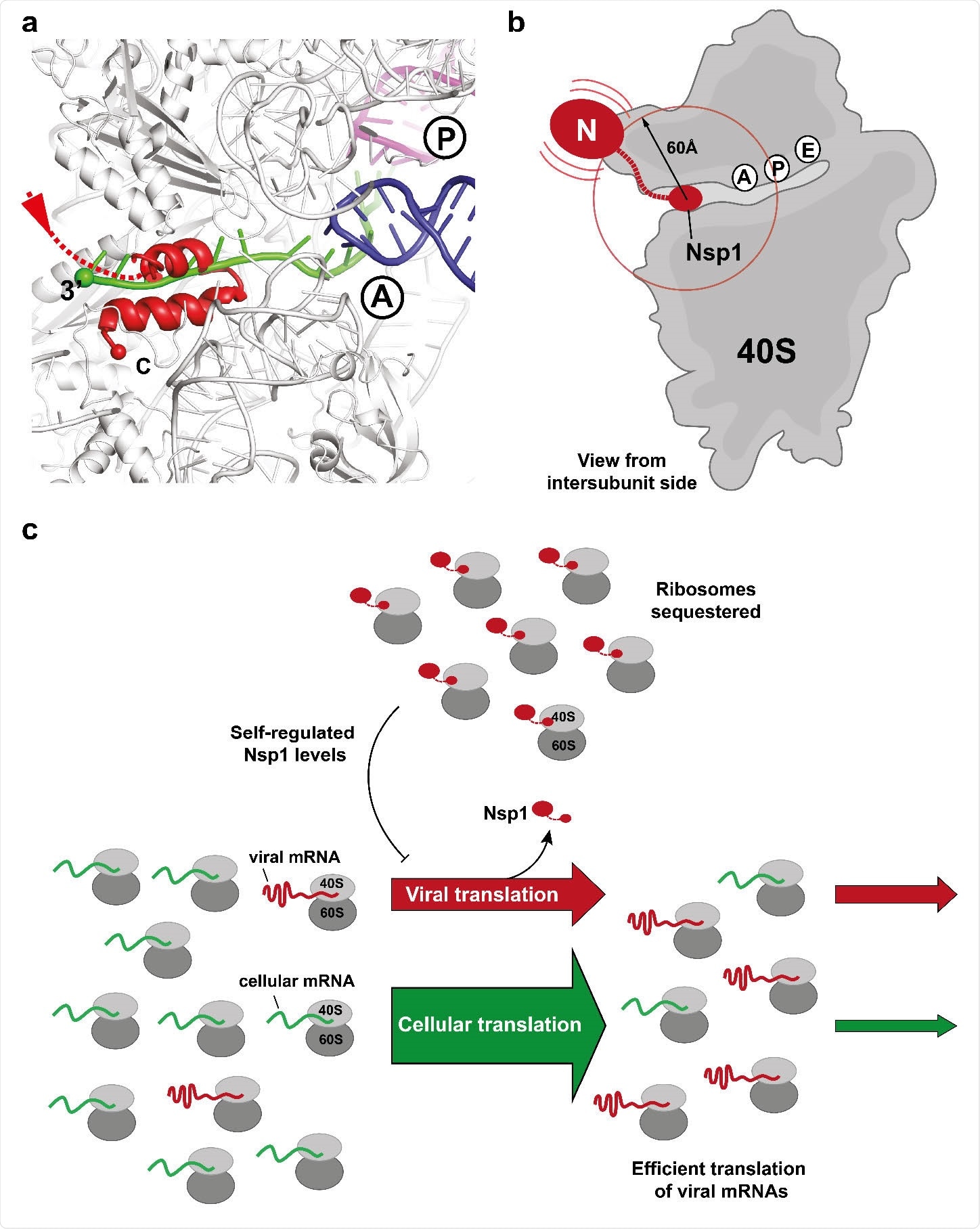
Binding of the C-terminal domain of SARS-CoV-2 Nsp1 to ribosomal mRNA channel prevents classical mRNA binding by sterical hindrance. (a) Superposition of canonically bound mRNA (green), A- (blue) and P-site (purple) tRNAs (pdb 6HCJ) reveals that Nsp1 (red) prevents classical binding of the mRNA at the entry site due to blockage. (b) Nsp1 binds via its C-terminus in proximity of the 40S mRNA entry site. Due to the flexible linker, the N-terminal domain can sample an area of ~60 Å around its attachment point (circle). (c) Model for translation inhibition by Nsp1. Upon viral infection and translation of viral genomic mRNA, Nsp1 acts as a translation inhibitor reducing the pool of ribosomes that can engage in translation. Under such ribosome-limiting conditions, viral mRNAs are translated with high efficiency.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Confirmation of Mechanism by Reporter Experiments And Mutations
They found that nsp1 almost completely inhibits translation of a luciferase reporter mRNA gene in a dose-dependent manner. Now, introducing some key mutations in helix 1 and 2, as well as in the KH motif, they found that this inhibition was abolished even at sixfold concentrations. They also failed to bind 40S ribosomal subunits, which indicates that the nsp1 has an affinity for binding the ribosome because of the C-terminal domain.
The study authors say this mechanism of inhibition may be found only in this virus and closely related beta-coronaviruses since only these have a conserved sufficiently long C-terminal that can bind the mRNA.
Differential Inhibition of Translation of Viral and Host mRNA
They also analyzed how the translation of reporter mRNA with viral 5’ UTRS was different from cellular 5’ UTRs, and how nsp1 affected both. They found that the former was five times more efficient, but both were inhibited at varying concentrations of nsp1. This is an important finding since viral RNA is translated in competition with cellular mRNA.
Overall, therefore, nsp1 is a general translation inhibitor, acting at the point of initiation. The mechanism is by sterically hindering the binding of mRNA by blocking the entrance of the mRNA channel. Further study is needed to understand how ribosomes are recruited to enable the viral mRNA to be efficiently translated.
One explanation might be that the potent inhibitory action of nsp1 leads to the tight binding of ribosomes and reduces the number of available ribosomes. In this restricted state, the ribosome will switch to the more efficient viral mRNA translation, because of the higher output achieved.
To enable this, the virus keeps nsp1 levels below the concentration that is required to inhibit viral mRNA translation, but enough to suppress the initiation from cellular mRNA. In fact, the researchers say, “Through this mechanism, we propose that Nsp1 would be able to inhibit global cellular translation particularly for mRNAs responsible for the host innate immune response, while the remaining ribosomes would still be able to translate viral mRNAs with high efficiency.”
As the levels of viral mRNAs increase, up to 50% of total RNA in the cell, they will shift more and more of the translation machinery towards viral proteins.
Implications
By identifying this key domain that interacts with ribosomes to control the cell’s response to the infection, attenuated vaccine strains will be easier to build. The study concludes, “Furthermore, these results provide an excellent basis for structure-based experiments aimed at investigating Nsp1 function in vivo by using viral model systems.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Schubert, K. et al. (2020). SARS-CoV-2 Nsp1 Binds Ribosomal mRNA Channel to Inhibit Translation. bioRxiv preprint. doi: https://doi.org/10.1101/2020.07.07.191676. https://www.biorxiv.org/content/10.1101/2020.07.07.191676v1
- Peer reviewed and published scientific report.
Schubert, Katharina, Evangelos D. Karousis, Ahmad Jomaa, Alain Scaiola, Blanca Echeverria, Lukas-Adrian Gurzeler, Marc Leibundgut, Volker Thiel, Oliver Mühlemann, and Nenad Ban. 2020. “SARS-CoV-2 Nsp1 Binds the Ribosomal MRNA Channel to Inhibit Translation.” Nature Structural & Molecular Biology 27 (10): 959–66. https://doi.org/10.1038/s41594-020-0511-8. https://www.nature.com/articles/s41594-020-0511-8.