As of mid-July, over 13.25 million people around the world have been reported infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), the respiratory illness responsible for the COVID-19 pandemic.
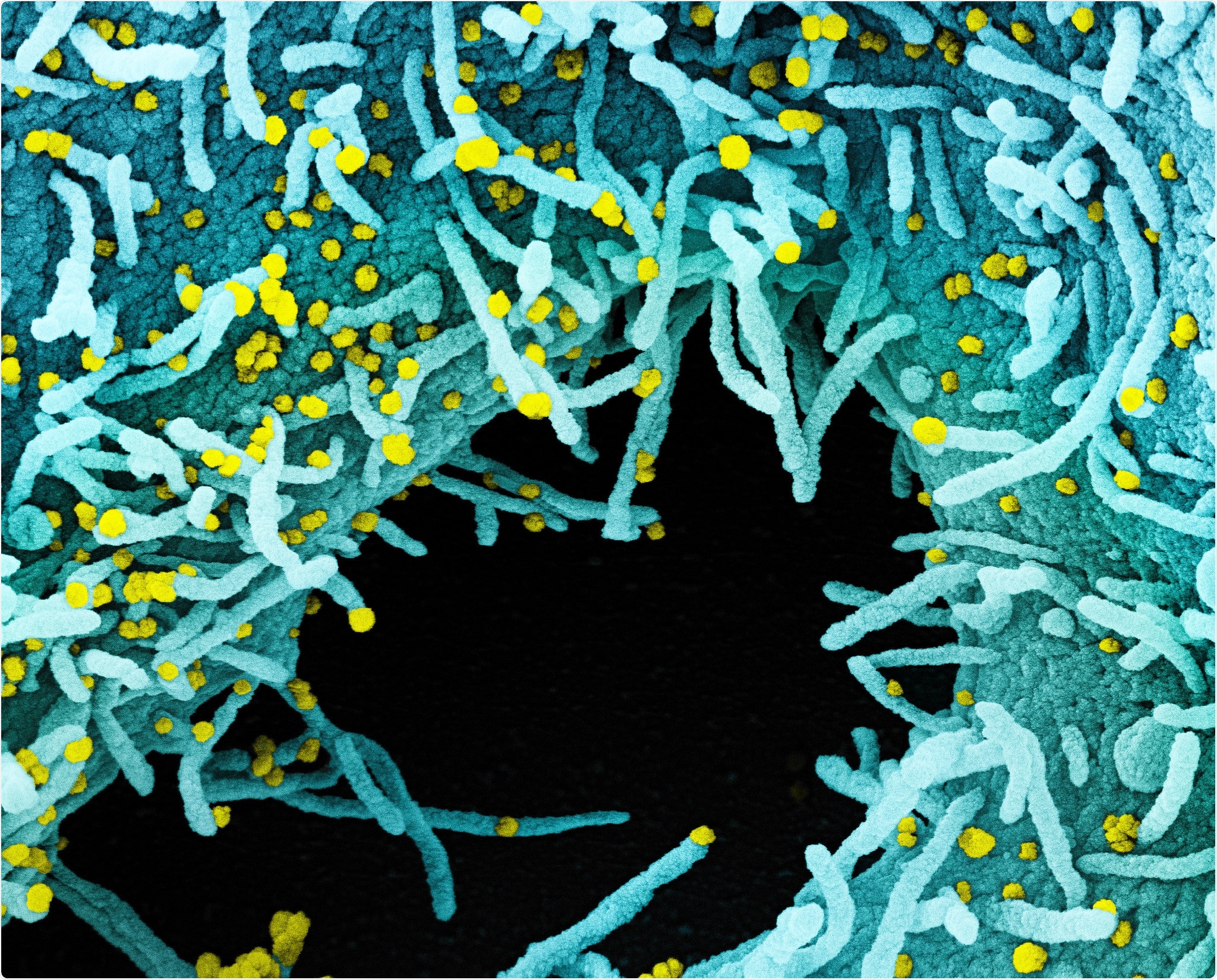
Novel Coronavirus SARS-CoV-2 Colorized scanning electron micrograph of a cell heavily infected with SARS-CoV-2 virus particles (yellow), isolated from a patient sample. The black area in the image is extracellular space between the cells. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This is why the World Health Organization (WHO) recently announced COVID-19 Technology Access Pool (C-TAP), where thirty countries and many international institutions pledged to find drugs and other health technologies in order to successfully tackle COVID-19. However, many potential drug candidates were screened well before that initiative, and some of them are showing great promise.
A novel class of hormone receptor modulators
Two compounds – currently designated as PT150 and PT156 – belong to a novel class of hormone receptor modulators with a primary clinical effect of blocking circulating cortisol, but with low-level glucocorticoid receptor (GR) agonism and androgen receptor (AR) antagonism at the cellular level.
PT150 is already a clinical-stage oral molecule (with completed phase 1 and 2 clinical trials) with a robust safety profile. Albeit initially used as an antidepressant, substantial antiviral activity against many viral families has been demonstrated for this agent. Naturally, the current pandemic was a perfect opportunity to take another look at it.
Hypothesizing that the activity against SARS-CoV-2 partly depends on the AR downregulation and modulation of angiotensin-converting enzyme 2 (ACE2) activity (i.e., receptor used for viral entry), the researchers from various institutions around the US decided to test this compound against SARS-CoV-2 in human bronchial epithelial lining cells.
A compounding effect of antiviral and immunomodulatory effects
This study has shown that T150 is a small molecule ready for clinical use, with a good safety profile and active investigational new drug application for COVID-19. The antiviral effects of PT150 observed in this study are primarily related to its capacity for AR antagonism; however, other mechanisms are unveiled as well.
"Given that hypercortisolemia is now suggested to be a significant co-factor for COVID-19 progression, we also postulate an additive role for its potent immunomodulatory effects through systemic antagonism of cortisol", study authors further emphasize.
Such a combination of antiviral and immune-modulatory effects that stems from this drug may yield substantial therapeutic value for all COVID-19 patients, as potential additive effects may undoubtedly reduce vulnerability to the infection.
Furthermore, the researchers found that the dose required for achieving anti-COVID-19 concentrations and SARS-CoV-2 viral suppression is relatively low, keeping it in line with obtainable drug concentration levels from already tested daily oral doses in clinical settings.
Pulse treatment and prophylaxis regimens
Older studies appraising the effects of P150 involved pulse treatments of giving the drug for two weeks, and two weeks without it. Such protocol was put forth to minimize the potential rise of Addisonian syndrome from the blocking the cortisol, or a rebound Cushing syndrome when the compound is withdrawn.
As neither syndrome was seen with such an approach, the safety of the dosing schedule was confirmed. Luckily, the natural course of a majority of SARS-CoV-2 infections is two weeks, so this protocol can be readily adjusted for treating early and milder infection (taking into account an estimated two-week duration of infection until clearance).
However, the use of P150 may also be considered in tailoring specific prophylactic regimens, which could be used by health care staff and other essential workers with a high risk of viral exposure. Still, some caveats do exist.
"PT150 would likely be contraindicated in that it would interfere with exogenous therapeutic glucocorticoid administration, recently shown to be beneficial in a study of dexamethasone in advanced stage COVID-19", study authors warn.
In any case, further studies are warranted to paint the full picture – in particular, to explore higher dose concentrations to determine adequate cytotoxic concentration for the compound in similar experimental models.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Sources:
Journal references: