Given the steady and menacing rise in both cases and deaths due to COVID-19 worldwide, scientists are still searching for efficient tools to diagnose the infection. One essential method of testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is serology, but this requires the supply of high-quality recombinant viral antigens.
The Spike Antigen
The SARS-CoV-2 spike antigen is among the most important of the viral antigens, with researchers focusing on it to produce vaccines, antivirals, and serological tests. Serological testing is essential for tracing cases, mapping contacts, undertaking epidemiological surveillance, and detecting asymptomatic patients, as well as teasing out the mechanism of antiviral immunity.
The spike protein comprises two subunits, S1 and S2, which mediate receptor binding and membrane fusion, respectively. This allows the virus to enter the cell. This protein is targeted by neutralizing antibodies, and the most common serological test for SARS-CoV-2 is the Enzyme-Linked Immunosorbent Assays (ELISA) based on the S protein.
This test is deservedly popular for its minimal cross-reactivity against the spike proteins of other coronaviruses in current circulation. Moreover, its intensity corresponds to the level of neutralizing antibodies.
The Study: Characterizing Low-Cost ELISA
The current study was based on identifying the key features that would enable the development of an inexpensive ELISA test that is based on the immunological reaction of an antibody to the S protein. The aim was to produce a test that would enable large-scale screening for infection in low-income countries.
The researchers focused chiefly on cutting the cost of test production by devising an optimal means of antigen production and making necessary changes in the method of antigen collection and sample processing. As a result, they were able to produce a test that will cost less than 50 cents per sample.
Producing Recombinant S Antigen
The researchers cultured the SARS-CoV-2 S protein in a stabilized prefusion state in cell culture, using a technique that would allow the S protein to be expressed stably and as a constitutive feature. This was by integrating the transgene into the genome of the cells in culture.
This adaptation results in better scalability as well as low-cost recombinant protein production. They also used co-transfection techniques with the S gene as well as an open-source plasmid that has a selection marker, to avoid having to wait for a synthetic gene to be constructed and shipped through pandemic-disrupted supply chains. The resulting recombinant cell line expressing a higher level of the S protein was thus produced within 24 days of transfection and has so far shown stable expression at up to 100 days.
This achievement makes it possible to “develop less costly, long-lasting batch-refeed or perfusion technologies for cell culture,” according to the researchers. They also succeeded in finding inexpensive growth mediums to support the robust growth of the cells and high levels of S protein production. Thus, they developed an entire workflow using low-cost methods to achieve the goal of increased cell density in culture with high S protein secretion.
The purification affinity chromatography (AC) resin used was more expensive than the initially planned ultrafiltration/diafiltration (UF/DF) filter, but became necessary due to the failure of the latter to remove smaller protein contaminants. However, they established that it could be used for over 30 cycles, which brings down the cost of its use.
They then set up an ELISA to detect anti-S antibodies in serum, plasma, and eluted whole blood samples, called S-UFRJ ELISA. They established the amount of highly purified S protein (from AC) needed to provide discriminating results between negative and positive samples, at 150 ng.
Evaluating Test Sensitivity and Specificity
The S-UFRJ ELISA was then used to test 210 negative and positive serum samples, with 66 samples from 38 symptomatic COVID-19 patients, 124 samples from before the pandemic, and 20 from COVID-19 negative people. They obtained 122 negative samples out of 124, for a specificity of 98%. Also, 53/66 samples were positive for IgG, for a lower sensitivity of 80%.
In comparison, only 46% sensitivity was obtained with a commercial IgG rapid diagnostic test (RDT) approved by the Brazilian health regulatory organization ANVISA.
They then retested the IgG-negative samples from the S-UFRJ ELISA by the rapid IgM test. They found that most samples that were negative for IgG were also negative for IgM, and those which were IgG positive in the first test were IgM positive by the rapid test as well. They, therefore, concluded that the two false negatives from symptomatic patients might have been samples collected at the beginning of the disease.
Increasing Positives with Increasing Duration of Symptoms
When the S-UFRJ ELISA sample results were mapped against the duration since symptom onset, they were more likely to be positive as the duration increased, resulting in the seroconversion of some individuals who were PCR-positive, scored negative on ELISA the first time but positive for anti-S IgG the second time. The rate of seroconversion to anti-S IgG by this test increased from 42% to 100% in direct relation to the day since symptom onset, and from the tenth day onwards, it was consistently above 90%.
An important finding was that the present test detects seroconversion sooner than the rapid test, which had a peak detection rate of 71% even at 20 days from symptom onset.
They also tested the neutralizing ability of COVID-19 patients by the plaque reduction neutralization test (PRNT). Samples with a high anti-S IgG titer had high neutralization titers.
Simplifying Blood Collection and Storage
The researchers also sought to overcome the traditional bottleneck of sample collection and processing in a clinical laboratory with refrigerated storage. They set up a simple system of fingerprick blood collection in filter paper strips. The use of dried blood spots on filter paper showed comparable results to serum testing.
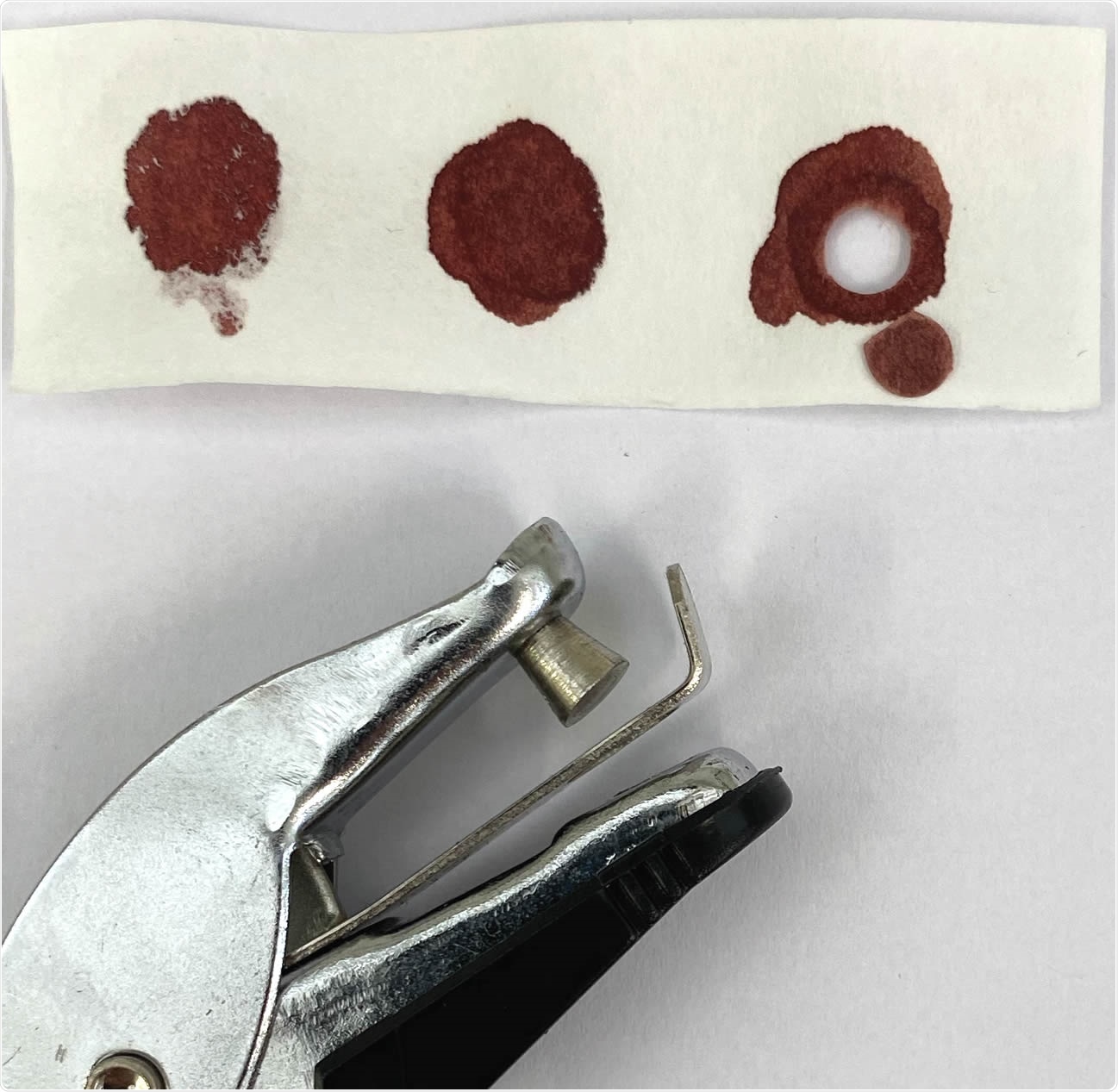
Dried blood samples (DBS) obtained by finger pricking with commercially available lancing devices. A 2.5 cm (W) x 7.5 cm (L) filter paper with three blood spots from the same volunteer and commercially available paper hole punching device was used to make DBS (arrowhead) from which blood was eluted for ELISA testing.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Implications
Thus, low-cost consumables, along with labor, transport, and equipment costs, should all fit within half a dollar per test, which is about 200 times less than the charge for tests now in use in the US. Another advantage is that blood spots in sealed plastic bags can be stored for 2 months at least but still return accurate serologic results.
Thus, the study sums up: “The S-UFRJ ELISA, comprising the use of eluates from whole blood finger pricks as samples, allows broad serological surveillance in populations regardless of their geographical and socio-economic aspects.” This will be invaluable to shape public health strategies and prevent new waves of the pandemic.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Alvim, R. G. F. et al. (2020). An Affordable Anti-Sars-Cov-2 Spike Elisa Test for Early Detection Of Igg Seroconversion Suited For Large-Scale Surveillance Studies In Low-Income Countries. medRxiv preprint. doi: https://doi.org/10.1101/2020.07.13.20152884. https://www.medrxiv.org/content/10.1101/2020.07.13.20152884v2
- Peer reviewed and published scientific report.
Alvim, Renata G.F., Tulio M. Lima, Danielle A.S. Rodrigues, Federico F. Marsili, Vicente B.T. Bozza, Luiza M. Higa, Fabio L. Monteiro, et al. 2022. “From a Recombinant Key Antigen to an Accurate, Affordable Serological Test: Lessons Learnt from COVID-19 for Future Pandemics.” Biochemical Engineering Journal 186 (August): 108537. https://doi.org/10.1016/j.bej.2022.108537. https://www.sciencedirect.com/science/article/pii/S1369703X22002066