From the first reported case of novel coronavirus disease in December 2019, the illness, now termed COVID-19 disease, fanned out across the entire world, and in March 2020, it was declared a pandemic. While China, where it originated, apparently stamped out the fire with Draconian restrictions on public mobility, other countries had longer epidemics with many more deaths.
Currently, hundreds of thousands of cases are being reported from the Americas, from India, and Russia, taking the total number of cases beyond 13.8 million, with a death toll nearing 600,000. Now, a study published on the preprint server medRxiv* in July 2020 reports on the low seroprevalence of antibodies to the virus, now designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), among blood donors in Wuhan, the Chinese city it all started in, and two other equally large cities in China. The authors point out that effective early management of the infection appears to have resulted in the efficient control of viral spread.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Testing for the new virus depends primarily on nucleic acid testing using upper respiratory swabs, in most cases. Early diagnosis could conceivably prevent the virus from spreading, but the vast majority of cases are mild or asymptomatic. These are diagnosed only by a systematic large-scale screening of contacts or simply testing at random among a specific population.
However, identification of the asymptomatic infection is vital to reduce the burden of viral spread and to understand the exact number of cases, which is essential to calculate the true case fatality rate of the infection. This appears to be possible only by using widespread serologic screening using specific antibodies.
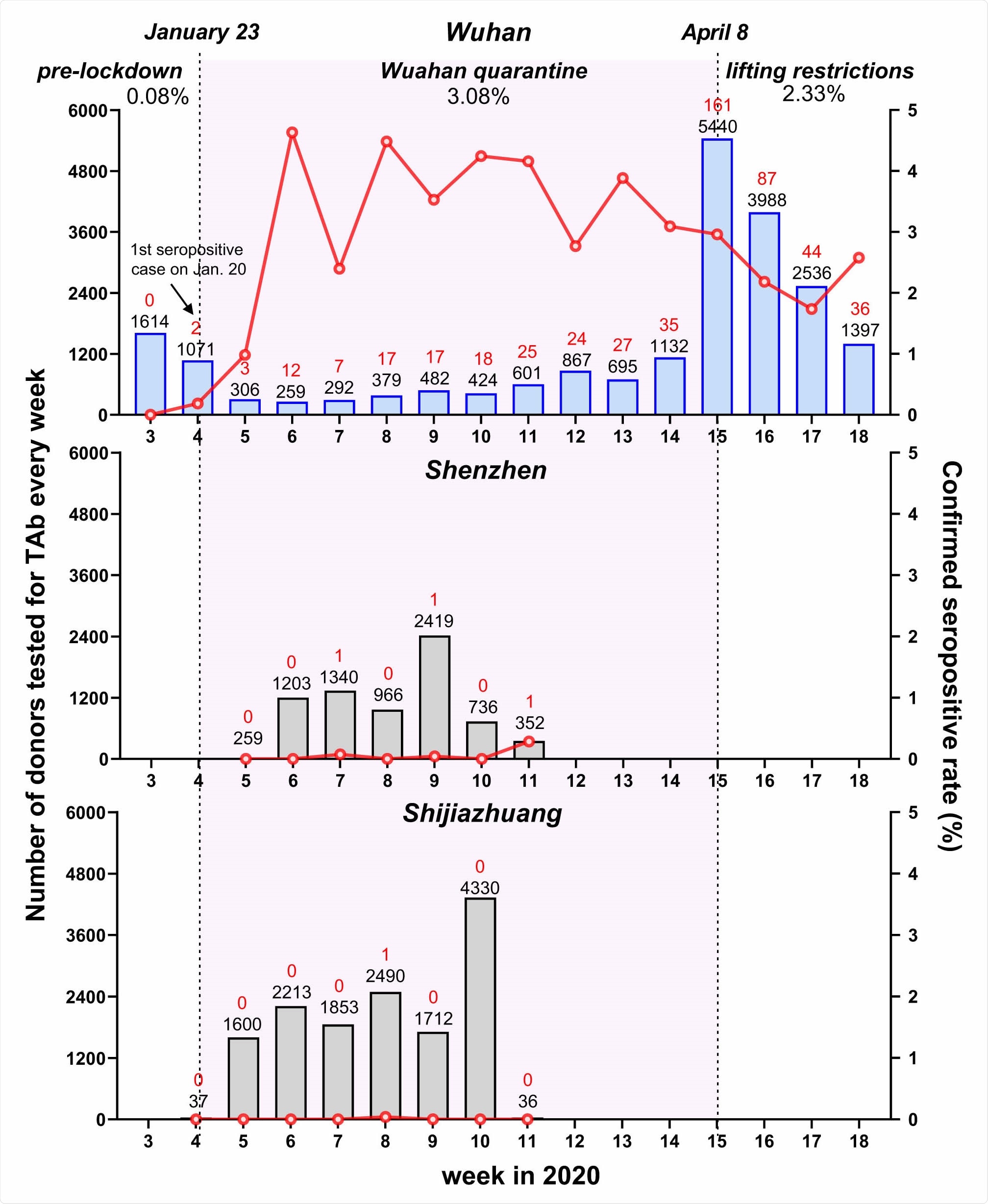
Weekly seroprevalence of SARS-CoV-2 antibody during different periods from January to April 2020 in the cities of Wuhan, Shenzhen and Shijiazhuang. The number of donors tested for total antibody (TAb) every week (the black numbers on the top of each histogram) is shown in histograms. The number of confirmed positive cases is shown in red numbers on the top of each histogram. The confirmed seropositive rate (number of ppNAT-confirmed donors/number of donors tested for TAb) in each week is shown in red lines. The first donor confirmed positive by the pseudotype lentivirus-based neutralization tests in Wuhan were on January 20, the fourth week of 2020. Lockdown of Wuhan City started on January 23 and on April 8, all the travel restrictions in Wuhan were lifted. The period of study in Wuhan is divided into three stages: pre-lockdown (Jan 15-Jan 22), lockdown (Jan 23- Apr 7), and lifting restrictions (Apr 8-Apr30). The confirmed seroprevalences of the three stages varied: two from 2607 donors were confirmed in the first stage (0.08%, 95%CI: 0.02%- 0.28%); 256 donations with confirmed serological evidence from 185 donors were identified from 7903 samples of 6004 donors, suggesting a seroprevalence of 3.08% (95%CI: 2.67%-3.55%) in the lockdown stage; After April 8, we further tested a total of 10,973 samples of 10,708 donors, and found out that 257 samples of 249 donors were confirmed SARS-CoV-2 seropositive (2.33%, 95%CI: 2.06%-2.63%). The peak of seroprevalence (4.63%, 12/259) occurred in the stage of lockdown.
The Pattern of SARS-CoV-2 Antibody Production
The earliest and most sensitive serologic test for COVID-19 appears to be total antibody (Tab), which returns positive from the second week after the onset of symptoms. At two complete weeks, all confirmed cases have positive Tab. In the second or third week, IgM and IgG antibodies appear, the first transient and the second sustained.
Prior studies on specific groups of asymptomatic individuals yielded a seroprevalence of 1.6% to 4.1%. The current study looks at antibody tests in asymptomatic groups in China.
The Study: Seroprevalence Among Chinese Blood Donors
The investigators looked at donors from three cities, Wuhan, Shenzhen, and Shijiazhuang, in central, south, and north China, with similar populations but different COVID-19 burdens. All donors who donated blood in the study period from January to April 2020 were included, at above 38,000.
Of these, the median age of the approximately 18,000 Wuhan donors was 33; of the almost 7,000 from Shenzhen was 36 years; and for the 13.500 from Shijiazhuang, it was 40 years. Most were male, at about 70%.
Antibody Testing
The researchers tested over 43,000 samples of the donated blood (some donated twice) for ‘Tab’, and found about 700 positives. Of these, about 590 were from Wuhan, about 30 and 60 from Shenzhen and Shijiazhuang, respectively. This comes to about 2.7% for Wuhan, and 0.4% for the other two cities.
The Tab positive samples were tested for specific SARS-CoV-2 IgG-RBD, IgG-N, IgM, and finally, a neutralizing assay against a pseudotyped virus (ppNAT). The seroprevalence was found to be 2.3%, 0.03%, and less than 0.01% in Wuhan, Shenzhen, and Shijiazhuang, respectively. This prevalence of asymptomatic infection among blood donors is lower than that seen in blood donors in European countries, which ranged from 3% to over 5%.
IgG-RBD positivity is seen in 96% of the 519 confirmed seropositive donation samples; 78% were positive for IgG-N and 66% for IgM.
Among the about 2,600 samples collected before the lockdown of Wuhan on January 23, 2020, there were about 1,600 and 1,000 samples from January 15 to 18, and January 19 to 22, respectively. Only two were seropositive at this time, for a blood donor seropositivity of less than 0.1%.
Once the quarantine of Wuhan began, the seroprevalence increased to 3%, while it dropped to 2.3% after the relaxation of lockdown on April 8, 2020. The highest seroprevalence was at the sixth week of 2020, from February 2 to 9, 2020, in blood donors from Wuhan, and has then been decreasing constantly.
However, the seropositivity was constantly low in blood donation samples from the other two cities, even during the quarantine period.
Antibody Markers and Neutralization Assay Markers
The researchers found that the average ppNAT titer went up as the Tab increased. The percentage of samples with a ppNAT ID50 above 20, which is the confirmatory level, was 38% at a Tab titer of 1-5. It increased progressively as the Tab titer increased from 81% with a Tab titer of 5-10 to 99% at a Tab over 15.
The ppNAT titer also went up as IgG-RBD, IgG-N, and IgM all increased. The closest correlation was with IgG-RBD. The N antigen appears to elicit the weakest antibody response.
The positive predictive value (PPV) of the Tab varies among different levels of seropositivity. While a positive Tab correctly predicted infection in 87% of Wuhan samples, the positive predictive value fell to 11% and 1.6% respectively in Shenzhen and Shijiazhuang, where cases were much fewer. This emphasizes that Tab-positive samples must be confirmed by other antibody tests.
The highest confirmatory value was with the addition of IgG-RBD testing, which improved the PPV to 99%, missing only 2.3% of true positives among Wuhan donors. However, this addition still cannot improve the PPV when the case burden is very low as with the other two cities.
Risk Factors for Asymptomatic Infections
The researchers searched for risk factors among the Wuhan donors since the other two cities contributed only three confirmed antibody-positive results. None of the confirmed cases among any of the donors in any city had a history of symptoms of COVID-19, making them asymptomatic cases.
Sex
The analysis shows that the independent risk factors for seropositivity include the female sex, which has 80% higher adjusted odds relative to males, and age. Males, however, have been shown to have more than double the mortality at 22% and 10%, respectively, and have a longer mean duration of hospitalization. Earlier research has shown that the case fatality rate is 1.64 times higher in males.
The IgG-RBD titer is also higher in females as a whole. All these findings imply that perhaps females are likely to have more chances for asymptomatic infection, while males are at greater risk for symptomatic disease. This is perhaps due to “estrogen receptor signaling mediated protections,” coupled with the increased prevalence of smoking among Chinese men, which may have resulted in more common lung damage.
Age
The adjusted odds increased from 10% higher among people aged 26 to 35 years old to 40% in the 36 to 45 years age group, 60% in the 46 to 55 years group, and fourfold higher for those above 55 years.
IgG-N antibodies were higher in older patients, while males tend to produce fewer IgG-RBD antibodies.
Implications
The findings suggest that Wuhan largely remained uninfected in the first wave of COVID-19 infection, due to the effective containment measures. This is also responsible, say the researchers, for the low antibody prevalence among donors in other cities.
The very low rate of seropositivity before January 23, 2020, indicates that since Tab appears about 10 days from infection, the virus is unlikely to have emerged in Wuhan’s donor population before January 10, 2020. This is supported by the seropositivity peak two weeks later, from February 2 to 8, 2020, followed by a fall during the quarantine of the city.
The seroprevalence continued to be low, at 2.3%, after the lockdown was relaxed. The researchers comment, “These data provided scientific evidence that demonstrated the effectivity in blocking viral community spread of strict public health measures performed in Wuhan.” It is worth noting; however, that other scientists have not always found Chinese data regarding the early phase of the outbreak to be reliable.
The study also brings out the need to confirm Tab-based testing using neutralization assays. The PPV of Tab testing depends on the case incidence of the population screened. When combined with neutralization results, the false-positives of Tab are around 0.4%, while the PPV value increases from 87% without additional IgG-RBD testing to 99% with it. This combination missed only 2.3% of true positives in the group of donors.
The investigators comment, “Neutralization confirmations are indeed required to exclude the false-positive reaction derived from immunoassays that may overestimate the real infection status, in particular for serological studies in a low-prevalence area.” The neutralization titer was also closely related to the IgG-RBD, so the latter can be used as a substitute if the former is unavailable.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources