We are beginning to understand the complexity of genetic diseases in general. These conditions include obesity, type 2 diabetes, and polycystic ovary syndrome (PCOS). All of these conditions are probably heterogeneous collections of disorders that have a final common path. They look similar but have different causes.
We conducted cluster analysis, which is a method for determining whether there are similarities in sets of data. The data analyzed in this study were hormone levels and metabolic parameters including glucose, insulin, and body weight measured in women with PCOS. We were able to show that there were two distinct clusters of hormone levels and metabolic traits. Further, we were able to replicate these findings in a second group of women with PCOS. Therefore, this was an objective assessment that demonstrated that there were subtypes of women with PCOS.

Image Credit: Alena Menshikova/Shutterstock.com
What is polycystic ovary syndrome (PCOS)?
The name PCOS is inaccurate because there are no cysts in the ovaries. The so-called cysts found with ovarian ultrasonography are actually the follicles that contain the oocytes or eggs. These follicles are arrested in development because of the hormone imbalance and appear as multiple small, round holes around the edge of the ovary. This finding is called the “pearl necklace” sign. PCOS is actually an endocrine syndrome, characterized by a slight increase in male hormone production by the ovaries and, often, the adrenal glands.
Frequently, there is a disruption of ovulation. This produces another key symptom - infrequent periods, often fewer than six to eight periods per year. Decreased frequency of menses is a clinical sign of not ovulating. The result of less frequent ovulation is infertility. The pituitary hormone, LH, that stimulates male hormone production by the ovaries, is frequently increased in PCOS, while the pituitary hormone that controls follicle maturation, FSH, is slightly reduced.
In about 1980, it was discovered that women with PCOS are also insulin resistant. I was just finishing my training as an endocrinologist around this time and I was fascinated by the association of metabolic problems and diabetes risk with a reproductive disorder.
How did you conduct your investigation into PCOS?
My whole research career has been focused on understanding these metabolic problems and the diabetes risk associated with PCOS. Through this research, it became apparent that there was very likely a genetic component of PCOS. PCOS is not a typical dominant or recessive kind of genetic condition known as a Mendelian disorder.
In contrast, PCOS is what is known as a complex genetic disorder that required genetic susceptibility interacting with environmental factors. The most common medical conditions that run in families, such as type 2 diabetes, bipolar disorder, and PCOS, are complex genetic disorders. For example, it is clear that you need to have lifestyle factors, in addition to genetic risk, to develop type 2 diabetes.
We have been able to apply new methods that have enabled genetic analysis on these types of complex genetic diseases. We started enrolling women with PCOS and their families in the mid-1990s. What really enabled the current study was that we characterized these women in detail and very precisely.
We measured a number of hormone levels, both reproductive and metabolic, in a highly reproducible way by using central laboratories and state-of-the-art techniques. We were able to recruit a large population of women with PCOS who were consistently and comprehensively characterized. This dataset turned out to be ideal for doing the cluster analysis.
How many people suffer from PCOS?
PCOS is exceptionally common. The classic and most severe form of PCOS, called NIH PCOS, characterized by the infrequent menstrual cycles and high male hormone levels, affects about 7% of reproductive-aged women worldwide.
The diagnostic criteria were broadened in 2003, in a conference Rotterdam, in the Netherlands. These criteria, which added ovarian appearance on ultrasound, are known as the Rotterdam criteria. In addition to NIH PCOS, the Rotterdam criteria added women with polycystic ovaries and high male hormone levels, but with normal cycles, and women with polycystic ovaries and irregular cycles but without high male hormone levels. About 15% of premenopausal women worldwide who fall into one of these PCOS categories.
Furthermore, PCOS tends to be very underdiagnosed because physicians do not know a lot about it. Women see, on average, two physicians before they get a diagnosis, and it often takes several years for the correct diagnosis to be made. Often, women diagnose themselves with information they find on the internet.
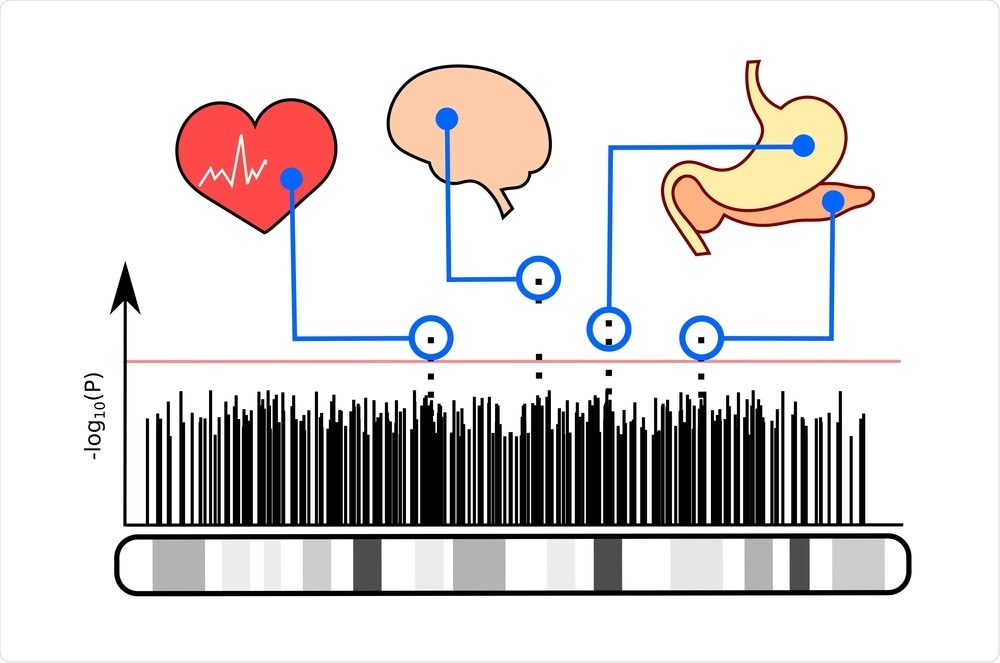
Image Credit: lanatoma/Shutterstock.com
How was genetic analysis carried out on the participants?
The genetic analysis used in this study was called genome-wide association. With the sequencing of the human genome in the mid-2000s, it became possible to map the entire genome. You could then investigate what areas of the genome were associated with your condition of interest. It has been a great way to uncover new biology about conditions because you do not need any preconceived notions about disease causes. You are just looking at the entire genome and asking, where are these signals that are associated with your disease?
GWAS have provided important insights into the pathways that cause PCOS. The first GWAS was published in late 2011 in a Chinese population. The Chinese investigators found that the genes that control how the gonadotropins, LH, and FSH, act on the ovary were significant signals.
In 2015, we published the first GWAS in European PCOS and found that the gonadotropin, FSH, was an important gene signal. This finding was confirmed in the second GWAS in European ancestry PCOS published shortly thereafter. These studies implicated gonadotropin secretion and action as important pathways in PCOS development. GWAS also discovered that a gene called DENND1A was associated with PCOS. Further research indicated that this gene is very important in regulating male hormone production.
We used the hormonal and metabolic data from our PCOS GWAS cases. We performed a cluster analysis to investigate whether there were subgroups within the PCOS cases. We then repeated the GWAS with these subtypes and discovered new genetic signals associated with the reproductive and metabolic subtypes. This finding was very exciting because it supports that the clusters identify biologically distinct groups of PCOS.
This study is the first, to our knowledge, to identify PCOS subtypes with unique genetic signals. We have discovered new genes regions, but we have a lot of work to do before we understand what genes in these regions are responsible for these signals and the function of the genes.
What does this research suggest are the possible subtypes of PCOS?
Our findings indicate that there are reproductive and metabolic subtypes of PCOS. Investigators have suspected that there are PCOS subtypes but no one has ever identified them by an unbiased data mining approach and independently confirmed that the subtypes thus identified were characterized by distinctive biologic features, in our study, by genetic differences.
It is important to note that all of the PCOS cases we studied had the severe NIH form of PCOS, characterized by infrequent menses and high male hormone levels. Future studies will investigate whether there are PCOS subtypes in the milder Rotterdam forms of PCOS.

Image Credit: Vitalii Vodolazskyi/Shutterstock.com
What were the characteristics of the ‘reproductive’ subtype?
PCOS women with the reproductive subtype have higher levels of the gonadotropin, LH, the pituitary hormone that is important in regulating male hormone production by the ovaries. LH levels are typically increased in the circulation in women with PCOS. It is ironic that the experts at the NIH and Rotterdam conferences on the diagnostic criteria for PCOS decided that LH levels should not be included in the hormones tested for diagnosing PCOS.
Our findings suggest that LH may indeed be a useful hormone for the diagnosis of PCOS. Unfortunately, at the time that decision was originally made, we did not have these data, but they show the value of scientific evidence compared to expert opinion for determining diagnostic criteria.
The reproductive subtype is also characterized by lower body weight and higher sex hormone-binding globulin levels. In addition, women with this subtype have higher testosterone levels, but these levels do not distinguish the subtypes as effectively as the high LH levels.
What were the characteristics of the ‘metabolic’ subtype?
The women who have the metabolic subtype have a higher BMI as well as higher glucose and higher insulin levels. We know that, overall, women with PCOS have about a 4-fold increased risk for developing type 2 diabetes. The metabolic subtype may identify the PCOS at the highest risk. If this is true, we could focus efforts to prevent diabetes on the metabolic subtype.

Image Credit: goffkein.pro/Shutterstock.com
What do your results suggest about a third group?
There were a number of PCOS cases that did not have a distinct subtype and were identified as “indeterminate”. It is interesting that an important gene signal in PCOS, in general, is FSHB, the gene for the gonadotropin, FSH, was associated with this indeterminate subtype. We need larger studies to understand the importance of this subtype.
How could these findings illuminate the possible causes and treatments of PCOS?
We found very strong gene signals associated with the subtypes. GWAS studies are designed to find common genes that are present in 5% or more of the population, and the signals that are associated with these genes are usually pretty weak. The associated genes increase the disease risk by 10 or 15%.
In this study, and we need to replicate these findings, the signals were much stronger, increasing risk by three to five-fold increases. We hope that it will be easier to map the genes associated with these signals. Gene discovery is key for identifying pathways that cause disease and that can be targeted with drugs. Genes with strong signals are very useful for predicting who will get the disease. PCOS cannot be diagnosed until a girl has gotten her periods.
However, we know that daughters of women with PCOS have features of the syndrome, like higher male hormone levels, well before they get their period. If we had a genetic test, we could say, "Your daughter is the one who is going to get this. And, hopefully, we will have therapies that are safe enough that we could use it to prevent the disease."

Image Credit: Branislav Nenin/Shutterstock.com
Do you hope your research will instigate more research into this area of women’s health?
Absolutely. I think what the limiting factor is, is funding.
Here in the United States, the National Institute of Health really is a conglomerate of different institutes that fund different diseases. There is just one institute, the National Institute of Child Health and Human Development, and they are sort of all of women's health, all of child health, all of disabled person's health.
Whether they are men or women, they are kind of not interested usually in women's health globally, and particularly women's and men's reproductive health. It is true for both men and women.
It is only at this one institute, competing with all these other meritorious research areas. And so, it gets a smaller share of a small pie and it is very sad.
There is an old saying here that was invoked early on when it was noticed that there were so many fewer studies of women, and women's health was really grossly underfunded. It is that you fund what you fear, and mainly these are guys, and mainly they are worried about heart disease.
What are the next steps for your research?
Our next steps are to replicate and confirm these genetic findings in additional populations of women with PCOS. This research should move quickly because we have collaborators who already have all the needed data in additional populations with PCOS.
The next step will be to find the genes are that are giving the signals and to look in diverse populations because it is very plausible that they are going to be different genes if you are African American than if you are of European ancestry. We are realizing that it is critical to understand genetics in diverse populations. We hope that this research will be translated into changing the way PCOS is diagnosed and, ultimately, treated.
It has been very gratifying for me to do research that has actually changed the way these patients are diagnosed and treated. I think that is the most satisfying thing for me as a physician and a scientist, to have had an actual impact on patient care. Obviously, ours is just one study, and we have to replicate it, as do others.
However, I think this type of research represents a new direction in medicine in which diseases are beginning to be classified based on scientific information rather than on expert opinion. Gene discovery is now a major way to develop new disease therapies because it tells us about what pathways are important so we can develop drugs to target that pathway.
Where can readers find more information?
https://icahn.mssm.edu/research/pcos
About Dr. Andrea Dunaif
Andrea Dunaif, M.D., is System Chief of the Division of Endocrinology, Diabetes and Bone Disease for the Mount Sinai Health System and the Lillian and Henry M. Stratton Professor of Molecular Medicine at the Icahn School of Medicine at Mount Sinai, New York, NY.
Dr. Dunaif graduated from Sarah Lawrence College and obtained her M.D. degree from Columbia University College of Physicians and Surgeons. She completed her training in Internal Medicine at Presbyterian Hospital in New York City and her subspecialty training in Endocrinology and Metabolism at the Massachusetts General Hospital in Boston. Dr. Dunaif’s first faculty appointment was at the Mount Sinai School of Medicine where she rose to the rank of Associate Professor of Medicine.
Before returning to Mount Sinai in 2017, Dr. Dunaif held a number of leadership positions in academic medicine, including the inaugural Director of Women’s Health at Brigham and Women’s Hospital and Director of Harvard Medical School’s Center of Excellence in Woman’s Health. She was the Charles F. Kettering Professor of Endocrinology and Metabolism at the Feinberg School of Medicine, Northwestern University where she served as Chief of the Division of Endocrinology, Metabolism, and Molecular Medicine for 10 years and Vice-Chair of the Department of Medicine for Research for 5 years.
Dr. Dunaif is an internationally recognized expert in endocrinology and women’s health. Her research on polycystic ovary syndrome (PCOS), the most common hormonal disorder of reproductive-age women, has shown that it is a leading risk factor for type 2 diabetes mellitus. Further, this research has revolutionized the treatment of PCOS with insulin-sensitizing drugs. More recently, she has made major advances in elucidating the genetic causes of PCOS and in discovering markers of PCOS risk in children and male relatives.
Dr. Dunaif has published more than 190 scientific articles and book chapters. She has edited four books. She has received numerous awards and honors including the Endocrine Society’s highest award for patient-oriented research, the Clinical Investigator Award, the Arnold Adolph Berthold Medal Prize from German Endocrine Society, and the Ricardo Azziz Career Award from the AEPCOS Society. She has been elected to the American Society for Clinical Investigation and the Association of American Physicians.
Dr. Dunaif received a Doctor Honoris Causa from the University of Athens Medical School. She is a past president of the Endocrine Society, the largest global organization for endocrinology, a former associate editor of The Journal of Clinical Endocrinology and Metabolism and Obesity and a past Chair of the National Institutes of Health Integrative and Clinical Endocrinology and Reproduction Study Section.