China is among the leading countries that are aggressively involved in developing vaccines for COVID-19, with one vaccine each from Sinopharm Group and Sinovac Biotech entering Phase 3 clinical development. The progress made by Chinese pharma companies in recent weeks indicates that they can surprise the world with their success in terms of the launch plan, says GlobalData, a leading data and analytics company.
Sinopharm and Sinovac (in collaboration with Brazil-based Butantan) have both reported encouraging results in the early phases of human testing for COVID-19. They now plan to test their vaccines in Phase 3 with a larger study population, at different locations across the globe.
With the growing number of COVID-19 infections and deaths across the globe, it is necessary to have a safe and effective vaccine as soon as possible to control the pandemic. However, there are no approved vaccines that are currently available. Vaccines from AstraZeneca, Moderna and Pfizer are the most talked about currently and are leading the vaccine development space. However, Chinese vaccines are equally progressing well compared to global players.”
Krishna Srinivasaraghavan, Pharma Analyst at GlobalData
Another Chinese vaccine developer The Institute of Medical Biology at Chinese Academy of Medical Sciences (IMBCAMS) has recently announced the initiation of Phase 2 clinical trial of a possible COVID-19 vaccine, following positive results from an ongoing Phase 1 study which recruited about 200 participants since May 2020. It is important to note that this vaccine has been granted conditional approval by the Chinese military on 25 June 2020.
In addition, IMBCAMS, in collaboration with CanSino Biologics, is developing an adenoviral (Ad5) based vaccine which is in Phase II. IMBCAMS announced plans to initiate China’s first safety trials on an mRNA-based vaccine, called ARCoV, in partnership with Suzhou Abogen Biosciences and Walvax Biotechnology.
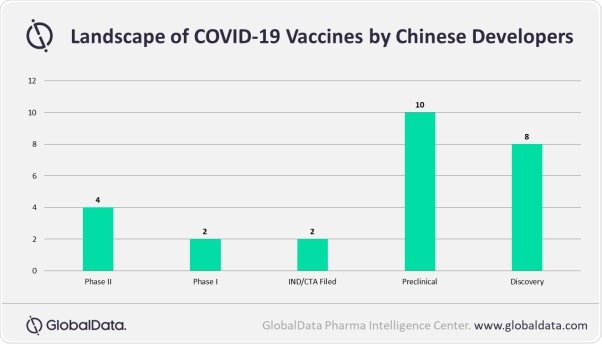
According to COVID-19 – Pharma Executive Briefing, 14 July 2020, more than 280 COVID-19 vaccines are under development across the globe, out of which only 6 vaccines are in the clinical development phase in China.
It is apparent that Chinese companies are working on different vaccine technologies. With ambitious progress in clinical trials, along with in-house manufacturing capabilities and infrastructure, Chinese companies are equally equipped to compete with global players such as AstraZeneca. However, their commercial strength will fall short of the global players and hence need partners to extend the commercial presence.”
Krishna Srinivasaraghavan