As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spreads throughout the world infecting millions, scientists are still frantically searching for an effective vaccine or antiviral.
One obstacle in SARS-CoV-2 vaccine development is the absence of a small animal model that undergoes infection with SARS-CoV-2 in a manner similar to humans.
Now, a new study published on the preprint server bioRxiv* in July 2020 presents an engineered transgenic mouse that reproduces the functional and clinical characteristics of severe human SARS-CoV-2 infection. This will allow the study of the disease process in humans, as well as help increase the efficacy of vaccines for SARS-CoV-2 and therapeutic drugs for COVID-19 disease.
The Importance of ACE2 in COVID-19
The SARS-CoV-2 virus gains access to the human host cells via the receptor molecule called the angiotensin-converting enzyme 2 (ACE2), used by both SARS-CoV and SARS-CoV-2 viruses. This receptor is found on a wide variety of cells, in the lung, the heart, the central nervous system, the kidneys, the gut, and in blood vessels as well as in fat tissue. Its physiological function includes negative regulation of the renin-angiotensin system and increased transport of specific amino acids.
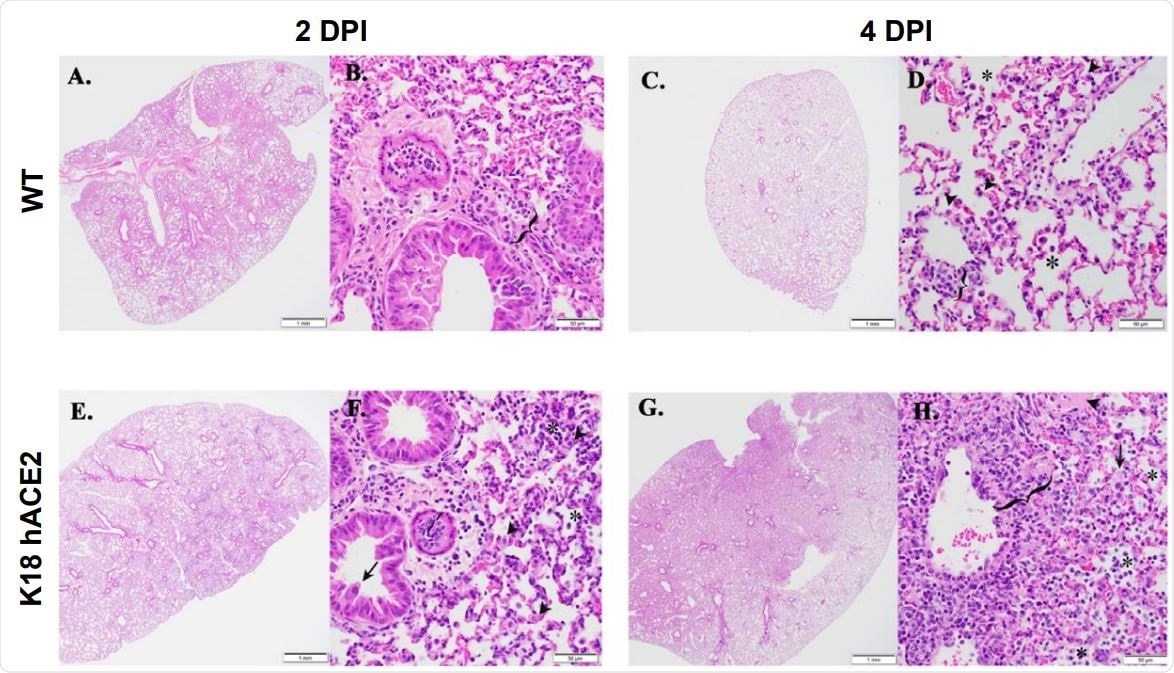
K18 hACE2 transgenic mice develop rhinitis, pneumonia with associated pulmonary inflammation after infection with SARS-CoV-2. (A-D and I-L) WT C57BL/6 mice. Minimal mononuclear and neutrophilic interstitial pneumonia in WT lung at 2-DPI (A, B bracket). By 4-DPI (5C, D) minimal alveolar histiocytosis (D, asterisk) pneumocyte type II cells (D, arrowheads), perivascular mononuclear inflammation (D, bracket) and rhinitis with low numbers of neutrophils (K, arrowhead) were variably observed. Lymphocyte aggregates in the lamina propria of the small intestine (I, asterisk). Mixed mononuclear inflammation with individual hepatocellular necrosis (J, arrowhead). Brain from WT 4-DPI was normal (L). (E-H and M-P) K18 hACE2 transgenic mice. Interstitial pneumonia (E, F) associated with alveolar histiocytosis admixed neutrophils and lymphocytes (F, asterisks), mild type II pneumocyte hyperplasia (F, arrowhead), bronchiolar syncytia (F, arrow), endothelial cells hyperplasia and vasculitis (F, bracket) by 2-DPI. Gut associated lymphoid tissue (GALT) with prominent germinal centers was observed (M, asterisk). Liver inflammation with variable amounts of individual hepatocellular necrosis (N, arrowhead). Greater lung involvement indicative of pneumonia (G), with inflammatory cellular accumulations and hemorrhage in alveolar spaces (H, asterisk) and interstitium (H, bracket), intra-alveolar fibrin admixed cellular debris (H, arrow), vasculitis (H, bracket), edema (H, arrowhead) by 4-DPI. Neutrophilic rhinitis observed at 4-DPI (O, bracket). Mild meningoencephalitis with vasculitis (P, arrowhead). Scale bars left images, 1 mm. Scale bars right images, 50 mm. DPI: Days post-infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Small Animal Models in COVID-19
If a small animal model could reproduce the respiratory infectious disease features seen in humans with severe COVID-19, this would help test out hypotheses related to the mechanism of disease as well as of drugs and vaccines. Not only are mice docile and easy to use in laboratory studies, they are also readily available, inexpensive, and capable of undergoing tests that will show how severe disease evolved in some people with COVID-19.
Previously, transgenic mice with human ACE2 (hACE2) expression have had promoters that allow the infection of the mice to an extent such that features of mild to moderate COVID-19 develop in many organs, as well as weight loss and immune responses. However, none of the models have developed fatal SARS-CoV-2 infection. The current study was focused on determining the susceptibility of the K18 hACE2 mice to fatal infection with SARS-CoV-2.
The Study: Transgenic Mice and SARS-CoV-2
The researchers worked with a strain of laboratory mice called K18 hACE2 transgenic mice, which can be infected with this virus, according to recent studies. The expression of the hACE2 in these mice is due to the presence of the cytokeratin 18 (K18) receptor. However, the critical point is that these mice express the receptor in their airway epithelium, just like human COVID-19 patients.
Early and Severe Disease
The researchers infected these mice with the virus to evaluate its suitability as an animal model for COVID-19 disease. They found that indeed the infected mice developed rhinitis, pneumonia, and lung inflammation. They rapidly lost weight and died within 6 days post-infection (DPI). Female mice began to lose weight at the third DPI, but male mice started to lose about 5% of their weight from 1 DPI. After the third DPI, all mice were noticeably and progressively ill and died by the sixth DPI.
High Viral Loads
The severity and time of death correlated well with the intensity of viral replication in the upper and lower respiratory tract and brain, measured at 2 and 4 DPI. The mice had ~1x103 PFU and ~1x104 PFU in the nose and the lungs, respectively. These were sustained at 4 DPI as well. Control mice had undetectable viral loads throughout the same period.
Early Cytokine Storm
They also found that the occurrence of cytokine storm, vasculitis as well as other features of local tissue damage. The nose and lung epithelium also showed a high level of viral nucleocapsid (N) antigen. The early cytokine storm, occurring at around 2 DPI in the lungs of the K18 hACE2 infected mice, with a marked rise in a number of cytokines relative to the controls. All the cytokines did not follow the same pattern, with some falling by 4 DPI.
The cytokines observed to rise included the inflammatory mediators TNF, IL-6, IFN-α, and IFN-λ), and those related to Th1 activation (IL-12, IFN-γ), TH17 (IL-17, IL-27) as well as to Th2 activation (IL-4, IL-10). Earlier reports indicate that IL-1 is the earliest to rise, and since this was not observed, earlier measurements should probably have been taken in this mouse model.
The cytokines that remained raised include TNF and the type I and III IFNs. Thus, these could be the promoters of progressive disease.
Reasons for Fall in Cytokine Titers
Significantly, other cytokines reduce even though the viral load is increasing, and the mice are showing progressively more severe wasting. The reasons for this could be the lymphoid infiltrate, which dampens the inflammatory response, the rise in Th2 cytokines which is anti-inflammatory, or the fibrosis-inducing IL-10 rise, which could also cause immunoparalysis by modulating the function of the neutrophils and monocytes recruited at the site of infection. Or the high IL-6 levels could enhance TNF activity in the acute phase, as well as recruit B cells to produce antibodies and induce fibrosis.
If one or more of these immunoregulatory mechanisms fails, an aberrant and dysregulated cytokine release might result, leading to a cytokine storm as seen in this mouse model.
Continuing Inflammation and Tissue Damage
Similarly, the sustained rise in the cell signaling molecule MCP-1/CCL2 could be a sign of continuing tissue toxicity and of the recruitment of more inflammatory cells to the site of infection. The resulting abundance of neutrophils and monocytes and subsequent vasculitis may be significant contributors to progressive and fatal COVID-19.
Here again, the researchers say, “the K18 hACE2 transgenic mice replicate cytokine and chemokine storm traits observed in humans.”
To measure systemic inflammation, chemokine titers in the spleen were assessed and found to be higher by 2 DPI, but not in the same pattern as those that arose in the lung. However, other tissues, including the spleen, failed to show evidence of a cytokine storm, showing this to be a local response.
Neurological Involvement
In the brain, Th1 and Th17 cytokines and chemokines rose by 4 DPI, which indicates that the virus may have reached the brain at this point. However, Th2 cytokines were dominant, probably upregulating IL-10 and thus modulating the inflammatory response. The occurrence of Th2 in the brain in control mice without evidence of the virus may mean that the virus can cross the blood-brain barrier. This could throw light on why some human infections are associated with neurological features. Interestingly, the Th1 and Th17 cytokines were higher in males than in females, while Th2 responses were higher in the latter. This may point to a propensity for neurological features in males relative to females infected with the virus.
The researchers also found that the hACE2 receptor was found in the lungs and nose, but also at high levels in the choroid plexus, which could indicate a potential route of spread of the virus within the central nervous system.
Conclusion: Mouse Model Could Help Unravel Disease Mechanism
The study concludes, “Our data provide evidence that K18 hACE2 transgenic mice represent an excellent animal model of SARS-CoV-2 infection and associated severe COVID-19 disease, providing the research community with a much needed small animal model to evaluate vaccines and/or antivirals for SARS-CoV-2 infection and associated severe COVID-19 disease in vivo.”
These findings will help to establish this mouse model which replicates the signs of terminal COVID-19 disease, and thus be an invaluable addition to non-human primates for preclinical testing purposes, since the latter do not show the signs of severe respiratory distress characteristic in humans.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources