By using specialized assays customized to detect specific receptor interactions, researchers from the Wellcome Sanger Institute in Cambridge UK find no evidence for direct binding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein to a transmembrane glycoprotein known as basigin. This study can be currently found on the bioRxiv* preprint server.
.jpg)
Novel Coronavirus SARS-CoV-2 Colorized scanning electron micrograph of an apoptotic cell (pink) heavily infected with SARS-CoV-2 virus particles (green), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
The global pandemic of coronavirus disease (COVID-19), caused by SARS-CoV-2, is accelerating with the increasing number of cases and deaths. Likewise, both fundamental and translational research on the causative viral agent is occurring at an unprecedented rate.
Since the start of the pandemic, it was clear that the precise identification of host receptors represents a pivotal step in mechanistically explaining COVID-19, offering important insights into SARS-CoV-2 cellular tropism and viral susceptibility.
A series of research studies published within the first months of the pandemic independently established that the same angiotensin-converting enzyme 2 (ACE2) receptor found to mediate SARS spike binding to human cells also mediates binding of the SARS-CoV-2 spike protein.
However, for previous coronaviruses that are closely related to SARS-CoV-2, a plethora of different host receptors were described, with numerous roles facilitating viral invasion. This made the research community question whether additional interaction partners (that are hitherto undiscovered) also play a role for the SARS-CoV-2 spike protein.
The basigin hypothesis
Among the most notable propositions for an alternate SARS-CoV-2 host receptor is basigin, also known as CD147 or EMMPRIN. This transmembrane glycoprotein (belonging to the immunoglobulin superfamily) was considered as a binding partner for the SARS-CoV-2 spike protein, with functional importance in viral invasion.
The early report identifying it swiftly translated to an open-label, clinical trial of meplazumab – a humanized therapeutic monoclonal antibody pointed against basigin. In a nutshell, its therapeutic use was linked to striking improvements in COVID-19 patients.
The possibility that basigin could indeed act as an auxiliary binding receptor for the virus has since then been speculated in several papers, possibly explaining the purported link between SARS-CoV-2 infection and hematological symptoms in the affected individuals.
Hence, Dr. Jarrod Shilts and Dr. Gavin J. Wright from the Cell Surface Signalling Laboratory of the Wellcome Sanger Institute at Cambridge, United Kingdom, decided to investigate the role of basigin as an alternate receptor for the virus.
Comprehensive binding experiments
"Our access to established tools and reagents from previous work studying the role of basigin as a host receptor in Plasmodium infection allowed us to rapidly investigate it as a SARS-CoV-2 receptor", explain study authors in their bioRxiv paper.
First, these researchers synthesized constructs to express the spike protein of SARS-CoV-2 recombinantly. In short, they have emulated already published, folded, and functional designs of spike constructs.
Next, they have sought to leverage the rather high sensitivity of direct biochemical binding assays to ascertain whether these methods could detect any traces of basigin binding. For that purpose, they have primarily used human embryonic kidney 293 (HEK 293) cell line and enzyme-linked immunosorbent assays.
Finally, after quality-testing the functionality of the constructs, the researchers have carried out a plate-based binding assay that utilizes the avidity gains of multimerized proteins in order to detect even exceedingly transient protein-protein interactions.
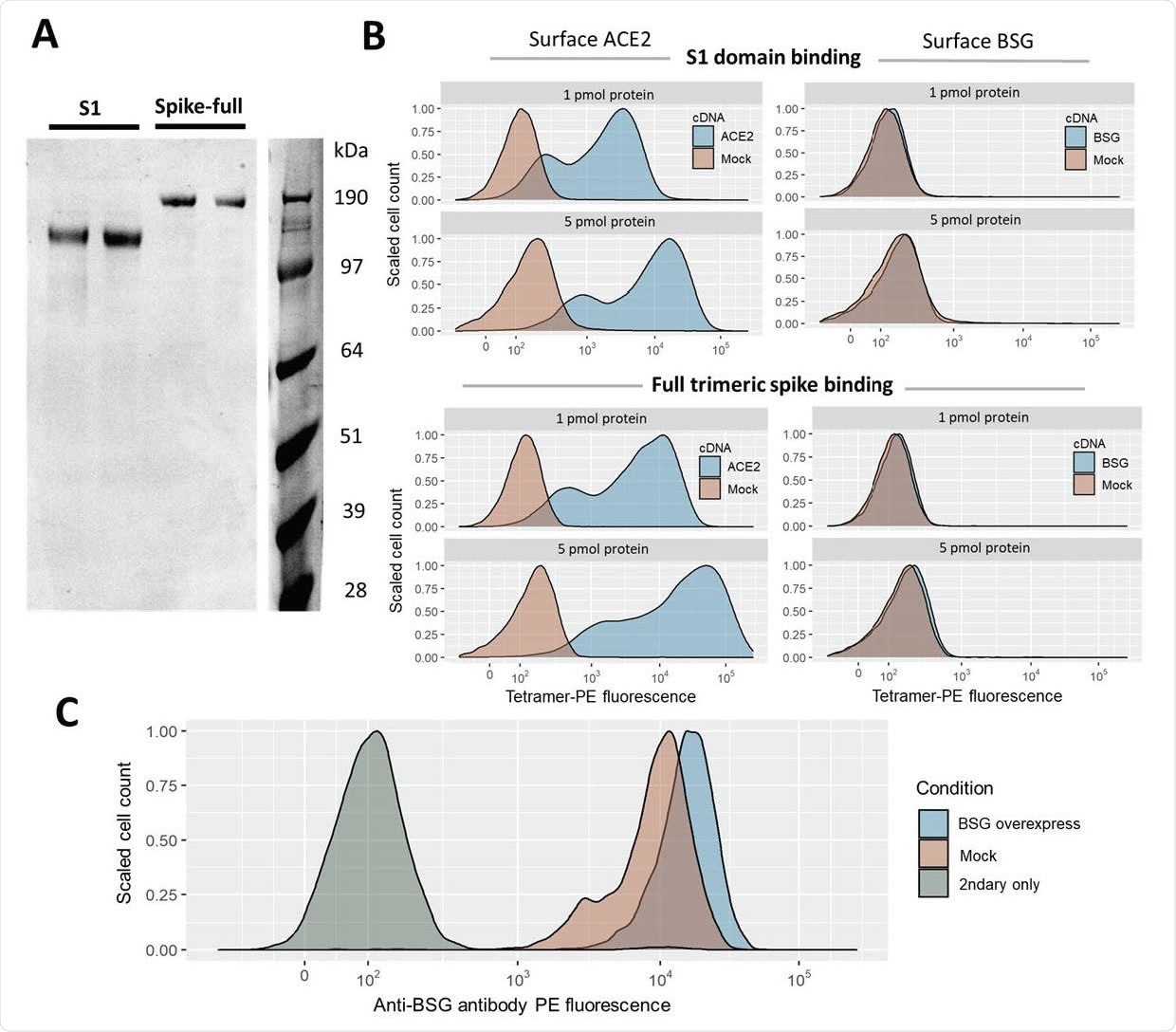
Gain of SARS-CoV-2 spike binding activity on human cells over-expressing ACE2 but not BSG. A. Expression and purification of the S1 domain and full ectodomain of the SARS-CoV-2 spike protein produced in human cell lines. Two independent preparations of purified spike were resolved by SDS-PAGE under reducing conditions and stained with Coomassie blue dye. B. Cells transfected with cDNAs encoding ACE2 but not BSG bind highly avid fluorescent SARS-CoV-2 spike tetramers. Flow cytometry fluorescence distributions of cells stained with tetramers made of biotinylated spike protein either using the S1 domain (top panels) or the entire ectodomain (lower panels) clustered around phycoerythrin-conjugated streptavidin. The stained HEK293 cells were transfected with cDNA to overexpress either ACE2 (left) or BSG (right). Mock-transfected cells are shown in red. Similar behavior to the data shown was observed in three separate tests. C. Transfection with BSG cDNA leads to upregulation of cell-surface BSG. Surface basigin levels on HEK293 cells labeled with anti-human BSG monoclonal antibody. BSG levels are compared to a negative control of secondary-antibody only.
Basigin not acting as a SARS-CoV-2 receptor
"Despite validating the functionality of all our reagents, we were unable to detect any binding in biochemical or cell-based assays for either common basigin isoform or either configuration or allele of the SARS-CoV-2 spike protein", study authors summarize their findings.
More specifically, in all tested configurations, the signal from basigin binding spike protein was indistinguishable from the background of non-interacting protein pairs, and substantially below the known interaction pairs.
Furthermore, recombinant forms of both the S1 domain and the entire ectodomain of the SARS-CoV-2 spike protein – known to directly bind ACE2 – did not interact with basigin expressed on the surface of human cells.
Implications and translational relevance
"Although our findings were negative, they nevertheless carry important potential implications to both our understanding of the basic biology of SARS-CoV-2 and efforts to translate knowledge of the virus' host receptors into therapeutics", caution study authors.
If these negative findings are confirmed, a clinical trial involving injections of anti-basigin monoclonal antibodies should be reinterpreted, since the patient benefit is then more likely explained by immune modulation (as opposed to directly blocking viral invasion). Furthermore, hypotheses that explain viral tropism via basigin binding may also necessitate closer scrutiny.
Therefore, more studies should be pursued to confirm the proposed mechanisms and resolve the uncertainty if SARS-CoV-2 can utilize any receptors beyond ACE2 during the infectious process – not just for basigin, but also for the myriad of other putative viral receptors.
Taking into account the dire need for identifying targets of SARS-CoV-2 that may lead to promising therapeutics, this study will allow more informed decisions regarding the translational relevance of this exact mechanism in our ongoing race to understand and manage COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources