Experts warn that the world may see repeated waves of the COVID-19 pandemic. However, scientists around the world are working hard to create a protective vaccine or antiviral, which could help to reduce the number of deaths and cases the next time around.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Advantages of Viral Vector Vaccines
The spike (S) protein of the SARS-CoV-2 virus is the major antigen that elicits neutralizing antibodies to the virus, preventing its entry into the host cell. This has been the primary focus of vaccine development efforts as a result. A number of viral vector vaccines have been reported expressing this protein. These have several advantages. Namely, they can be used as live or inactivated vaccines, and the regimen can be tweaked to induce predominantly Th1 or Th2 type immune reactions. Moreover, they can be developed and handled in Biosecurity Laboratory Level 2 (BSL-2 ) conditions. Finally, they can be cultured in high numbers using cell cultures or other specific hosts.
Several viral vector vaccines are being developed for the current virus, including measles virus and Modified Ankara vaccinia. The present study used the NDV vector, which has a significant additional advantage that it can be amplified in embryonated chicken eggs. Since many firms manufacturing the flu vaccine all over the world are already using egg-based methods, this gives them the capacity to switch over to making the new vaccine in hundreds of thousands of doses.
This results in higher yields and, therefore, lower manufacturing costs, which inevitably means lower end-user costs per dose.
The NDV Viral Vectors
The NDV is a paramyxovirus and is usually not associated with human illness except for rare conjunctivitis or flu-like symptoms. Secondly, since NDV is not a human pathogen, the presence of cross-reactive antibodies within the human vaccinee is unlikely, allowing for proper antibody response. And finally, the LaSota and other strains of the NDV have been used to create a variety of oncolytic viruses that have been run through clinical trials, giving it an excellent safety record.
The researchers produced two types of NDV vectors expressing the wild-type spike protein of SARS-CoV-2 and a prefusion S-F chimera. The latter consists of the spike ectodomain, with three arginines removed to keep the protein stable in its prefusion conformation. This was equivalent to removing the polybasic cleavage site.
It is also anchored more firmly to the membrane surface of the NDV particles by replacing the transmembrane domain and the cytoplasmic tail of the original S protein with those of the fusion protein of the NDV.
They found that both the wild-type and the chimeric S protein were well expressed as transgenes, inside the infected cells, encoding the WTS and S-F on the surface of the NDV particles. All the viruses grew to a titer of ~108 FFU/ml within the embryonated chicken eggs.
However, the researchers showed that the insertion of a large transgene still allowed the virus to be grown inside the chicken eggs. They also observed that S-F was incorporated into the viral particles much better than the WTS, but the S protein was successfully expressed by infected cells. The WTS still containing the polybasic cleavage site was shown to be cleaved entirely, showing only the presence of S1, but the S-F was preserved in the prefusion conformation.
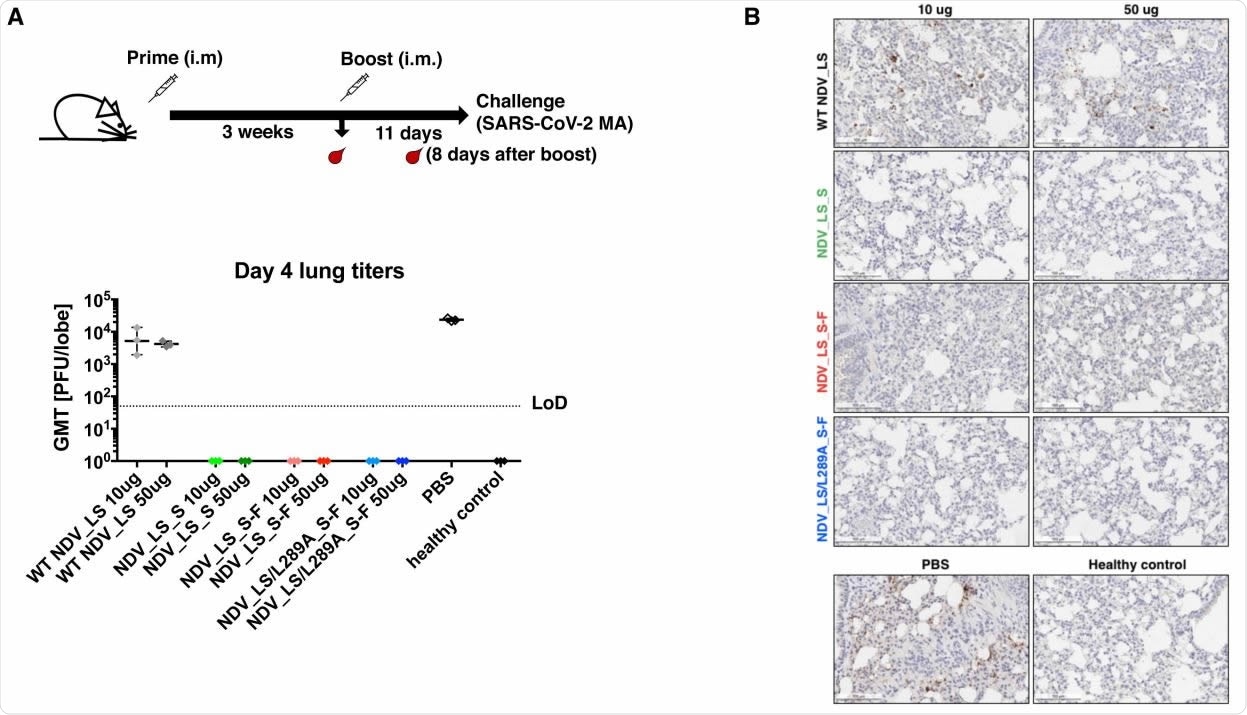
NDV vector vaccines protected mice from the SARS-CoV-2 challenge (A) Viral titers in the lungs. All mice were infected intranasally with 104 584 PFU SARS-CoV-2 MA strain except the healthy control group, which was mock infected with PBS. At day 4 post-challenge, lungs were collected and homogenized in PBS. Viral titers in the lung homogenates were determined by plaque assay. Plaque-forming units (PFU) per lung lobe was calculated. Geometric mean titer was shown for all the groups. LoD: limit of detection. (B) Immunohistochemistry (IHC) staining of lungs. A SARS-CoV-2 NP specific antibody was used for IHC to detect viral antigens. Slides were counterstained with hematoxylin. A presentative image was shown for each group. The brown staining indicates the presence of NP protein of SARS-CoV-2.
Proof of Concept: Mouse Immunization Assay
The study also included the intramuscular injection of three live NDV S-F expressing vectors into mice, through a prime-boost regimen over three weeks. This route was chosen because live NDV does not replicate well in the muscle and fails to cause symptomatic infection in mammals.
Both the WTS and the S-F constructs resulted in the production of high levels of binding and neutralizing antibodies by the mice. Moreover, when the mice were exposed to a strain of SARS-CoV-2 adapted to this animal model, they were found to be completely immunized against the virus. On day four, following the challenge, there was no detectable viral titer or viral antigen in the lungs.
The study concludes: “We have developed promising cost-effective SARS-CoV-2 vaccine candidates using the NDV LaSota strain as the viral vector, which could be generated to high yield under BSL-2 conditions.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources