Even as the COVID-19 pandemic claims increasing numbers of patients, testing numbers are rising in most nations at the leading edge. However, the current reverse transcriptase-polymerase chain reaction (RT PCR) testing is often unavailable and takes up to several days for results to arrive.
Apart from the delay inherent with the use of RT PCR on nasopharyngeal or oropharyngeal swabs, the procedure is unpleasant for most people, and some scientists question its sensitivity. Thus, a new and more rapid test with better sensitivity and increased prognostic significance would be welcome. This would help to triage patients for hospital admission or close surveillance based on the predicted severity of the disease at the time of presentation.
The only currently available test that meets these standards is the chest computed tomography (CT) scan to detect pneumonia, but this is neither inexpensive nor very specific, though it does have high sensitivity. Moreover, it is available only in specialized centers.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Blood Clotting in COVID-19
Many papers have acknowledged the presence of widespread blood clots and their part in causing severe COVID-19 disease. However, extensive hemorrhage can also worsen the outcome in patients with these infections, and this phenotype has also been recognized. The common factor in both these end-of-spectrum manifestations is the realization that these are early and late phases of the same disordered clotting process.
In the initial phase, excessive clots are formed, and partial fibrinolysis occurs, causing a rise in D-dimer levels that have been shown to b characteristic of poorer outcomes. As clot formation proceeds and extends, fibrinogen and von Willebrand factor (vWF) levels fall, along with the platelet count. This leads to the second phase of excessive bleeding, including disseminated intravascular coagulation, which may be a life-endangering manifestation.
In recognition of this, researchers have suggested the use of anticoagulant therapy earlier in the disease, with careful monitoring of the patient via serum coagulation parameters, to improve the outcome.
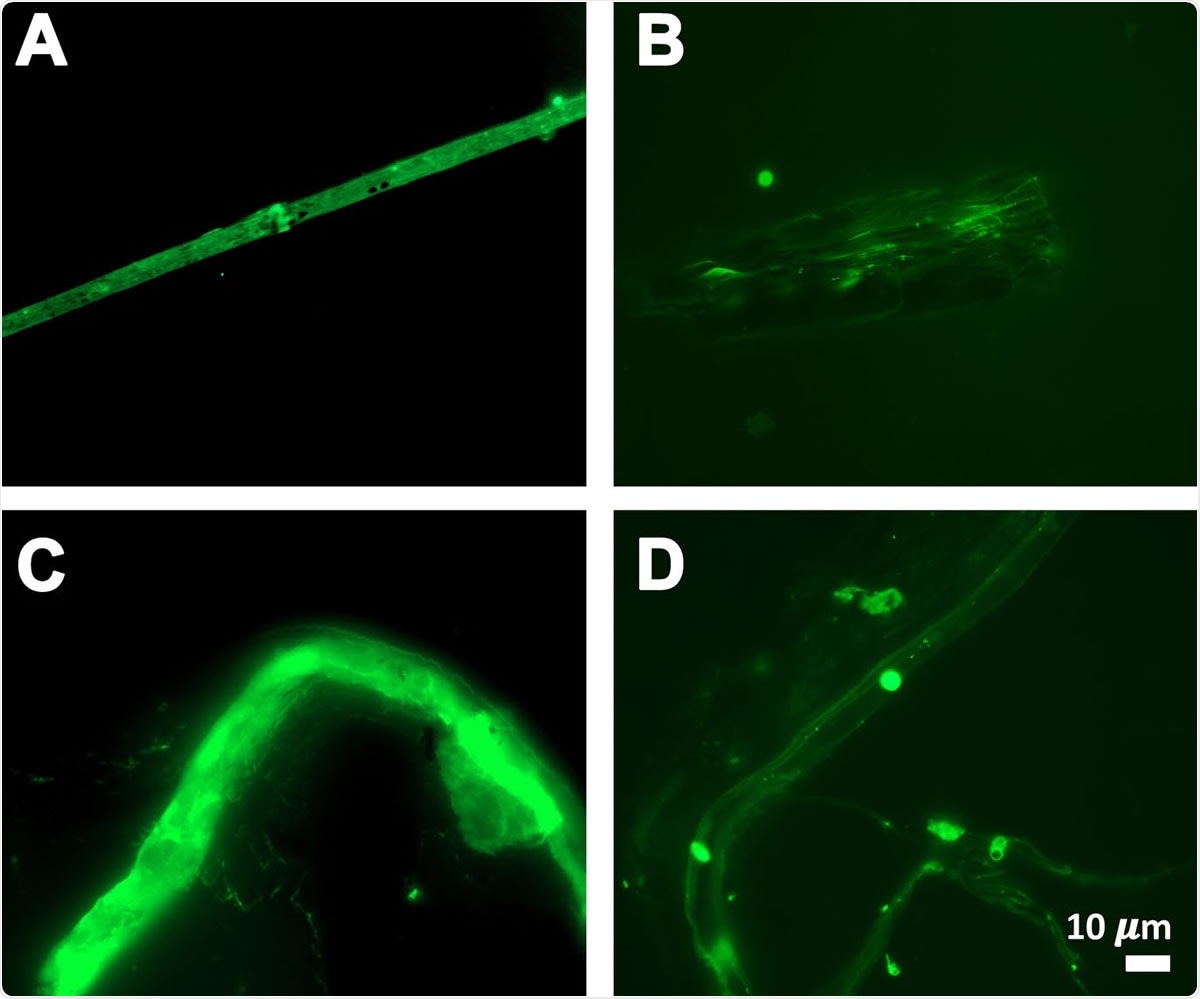
Fibrous or cellular deposits in the plasma smears of COVID-19 patients
Nature of Clots
In severe COVID-10, clotting disorders are associated with other signs of extensive tissue injury such as high ferritin levels and the occurrence of a cytokine storm later in the course of the disease. The presence of excessive iron is an established trigger of amyloid clot formation, and these signs are not peculiar to COVID-10. Rather, they occur in all inflammatory conditions.
The researchers hark back to their earlier research, which showed that blood fibrinogen could clot into an abnormal and amorphous form called amyloid clots when exposed to certain chemical compounds in microbial cell walls, and in several chronic inflammatory conditions. Amyloid clots are detected using thioflavin T, a fluorescent dye, or Amytracker stains. In published research, these clots were formed in vitro, using plasma to which thrombin, another clotting factor, had been added.
The Study: Amyloid Clots in PPP
The researchers used 20 samples from 11 male and 9 female COVID-19 patients, collected before any treatment was begun. These samples were stored as platelet-poor plasma. There were also samples from 10 controls of similar age and sex, collected from the plasma bank. None of the controls were smokers; all had normal C-reactive protein and were not on anti-inflammatory medication. The average age was about 50 years and 49 years for patients and controls.
Among the infected patients, 10/20 were eventually sent home for observation after drawing blood samples. For moderate or severe illness, CT chest scans were carried out. Therefore, CT scan performance was used to classify patients in the study as having mild, moderate, or severe illness. Fluorescent microscopy was used to detect amyloid clots in PPP from patients and controls.
In contrast to the clots found in their earlier study, the plasma from COVID-19 patients showed the same type of clots, but the investigators comment, “the signals were so massive that they were essentially off the scale.” And in fact, this plasma contains a huge amount of already existing amyloid clots without the need to add thrombin.
Implications
The researchers say this is another of the unique clinical features of COVID-19. It explains both why widespread microclots are formed and how it should be prevented as a primary treatment step, using anticoagulants.
The microclots need close monitoring via thromboelastography (TEG) or similar techniques to prevent the patient from progressing to the second phase, which is characterized by hemorrhage. Here, TEG has the advantage of offering the ability to study both whole blood and PPP for clotting parameters.
Whole blood TEG allows the evaluation of clotting in the presence of platelets and fibrinogen, the initiator of the clotting cascade. PPP TEG allows only plasma protein-mediated coagulation to be tested.
Although the current study used fluorescence microscopy, TEG is a useful option, being available at point-of-care, inexpensive, and reliable. The researchers describe the assay they used as being easy, quick, and cheap, taking only 40 minutes, including incubation time of 30 minutes. They opine, “This, therefore, provides a rapid and convenient test for COVID-19.”
TEG is also helpful in detecting the extent of clot breakdown. Some researchers have reported a complete suppression of fibrinolysis at 30 minutes by TEG, and this is invaluable in predicting the occurrence of thromboembolism in COVID-19. The amyloid nature of the microclots frequently observed in COVID-19 patients explains why this fibrinolysis is complete and why hypoxemia occurs so often, emphasizing the need to prevent it by early therapy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources