The team reports that SARS-CoV-2-specific IgG responses were sustained both at the site of infection and systemically in most patients with coronavirus disease 2019 (COVID-19) and could be detected in both saliva and serum samples.
Anne-Claude Gingras and colleagues say the findings suggest that saliva samples could be used as an alternative biofluid for monitoring immune responses to SARS-CoV-2.
Characterizing the nature and kinetics of salivary antibodies as soon as possible among infected, contact-traced individuals will also be critical to establishing any correlates of protection that might impact COVID-19 disease progression, they add.
A pre-print version of the paper is available on the sever medRxiv*, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antibodies play a vital role in virus neutralization
The antibodies generated following a viral infection play a vital role in neutralizing the pathogen and can protect re-infection in the future.
“Understanding their durability and their system compartmentalization across a diverse population are critical pieces of information informing our ability to monitor seroprevalence in communities, to select plasma donors for treatment, and to design vaccines against COVID-19,” write Gingras and team.
In the case of COVID-19, the antibody response to SARS-CoV-2 in the blood has been widely studied. Still, comparatively little is known about the mucosal immune response and how this relates to the systemic antibody response.
Whether the antibody response to SARS-CoV-2 lasts is an “extremely important issue”
The antibody response to the SARS-CoV-2 spike protein is particularly important because this protein harbors the receptor-binding domain (RBD) for the human host cell receptor angiotensin-converting enzyme 2 (ACE-2).
Neutralizing antibodies have been shown to target this RBD, and many studies have reported that IgG antibodies against spike and the RBD can be detected in most patients 10 to 11 days following symptom onset.
However, whether this SARS-CoV-2-specific IgG response is long-lasting remains unclear and is a subject of ongoing debate.
“Examination of different biofluids from multiple cohorts, and attention to the antigens tested, is required to resolve this extremely important issue that has high relevance to vaccine design,” said Gingras and colleagues.
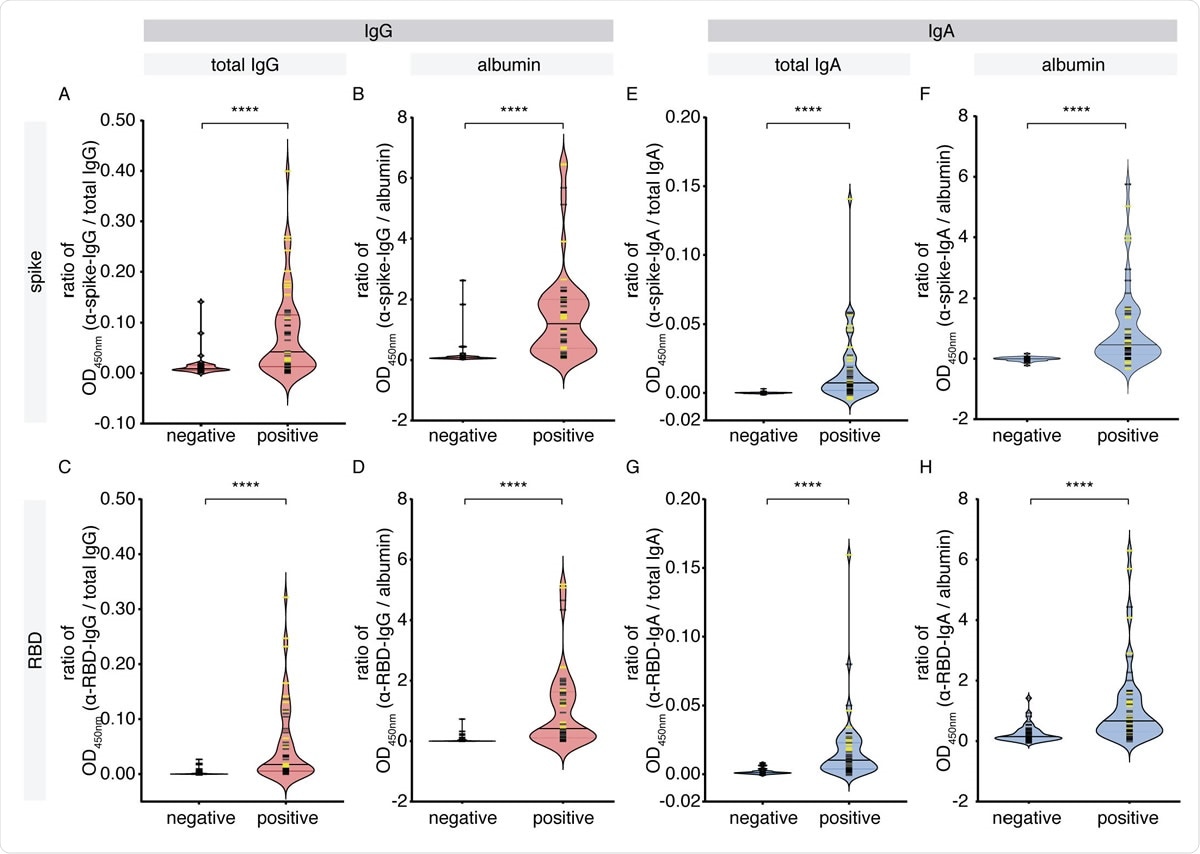
IgG and IgA levels against SARS-CoV-2 antigens in the saliva of cohort 1. A pilot cohort of COVID-19 patients was tested for the presence of IgG and IgA antibodies to SARSCoV- 2 spike and RBD antigens in the saliva, comparing with age- and sex-matched unexposed negative controls collected locally. (A-D) Antigen-specific (anti-spike, anti-RBD) IgG levels normalized by total IgG or albumin. (E-H) Antigen-specific (anti-spike, anti-RBD) IgA levels normalized by total IgA or albumin. Yellow bars denote saliva samples collected at an unknown dilution. Solid bars denote the median. Mann-Whitney U test for significance was performed. **** = p < 0.0001.
Little is known about antibody responses in the oral cavity
Furthermore, very little is known about the antibody response that occurs locally, at the infection site, the authors add. However, since the virus initially replicates in the naso- and oro-pharyngeal tracts of the upper airway, the antibody response in the oral cavity is probably important in terms of influencing the course of infection.
Still, despite the oral cavity being a known site for SARS-CoV-2 replication, the authors say studies examining the antibodies generated at this site have been limited.
To investigate, the researchers developed enzyme-linked immunosorbent assays to detect SARS-CoV-2 antibody responses to the full-length spike protein and its RBD in serum and saliva samples taken from acute and convalescent patients. The participants had been diagnosed with COVID-19 between three and 115 days following symptom onset.
Antibody stability levels in 496 serum samples and 90 saliva samples were compared with stability levels in SARS-CoV-2-negative control samples.
IgG antibodies levels were still stable in both biofluids after three months
The team reports that antigen-specific IgG antibodies were readily detected in both types of biofluid, with peak levels observed at 16 to 30 days following symptom onset.
The SARS-CoV-2-specific IgG levels were still relatively stable in both sample types up to 115 days following symptom onset (when the study period ended).
Importantly, the IgG responses against both full-length spike and its RBD were strongly correlated, suggesting that saliva could serve as an alternative to serum for monitoring the systemic immune response to SARS-CoV-2.
Characterize salivary antibodies in the future will be critical
“Our data show that a durable IgG response against SARS-CoV-2 antigens is generated in both the saliva and serum in most patients with COVID-19,” write the researchers.
“Given that SARS-CoV-2 initially replicates in the oro- and nasopharyngeal tracts, in the future it will be critical to characterize the nature and kinetics of salivary antibodies at the earliest time points post-infection in contact-traced individuals in order to determine if there are correlates of protection that impact viral setpoint and COVID-19 disease progression,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources