The current COVID-19 pandemic is a primarily respiratory illness, manifesting with severe hypoxia and often progressing to acute respiratory distress syndrome (ARDS) and death. However, it is also known to be linked to a wide range of cardiovascular (CV) manifestations that are associated with a very poor prognosis.
Cardiovascular Manifestations and Outcomes in the Setting of COVID-19 Disease

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
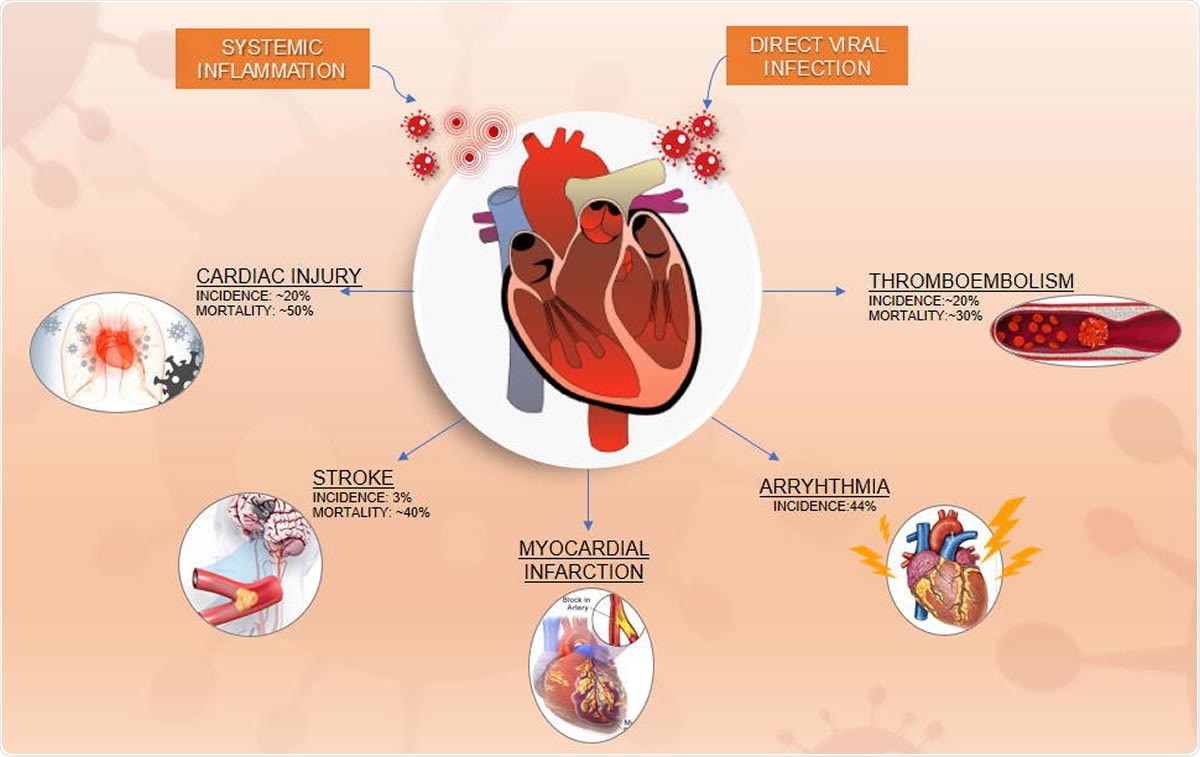
Mechanism of CV Involvement in COVID-19
Most investigators consider that the CV manifestations in this syndrome are due to direct viral infection of the myocardium, coupled with systemic inflammation. Both cardiac injury and inflammation are known to be common in this condition and may be the result of the infection of the myocardial cells.
Most scientists think that the most important route of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into the host cells is through the angiotensin‐converting enzyme 2 (ACE2) receptors, which are profusely expressed in the heart and lung tissue. Another often-presented theory is that the systemic secretion of pro-inflammatory cytokines leads to indirect cardiac injury. These include interleukin-1 (IL-1), beta interferon-gamma (IFN-γ), tumor necrosis factor (TNF)-α, and interleukin-6 (IL-6).
The role of systemic inflammation in causing cardiac injury is supported by an autopsy report of a COVID-19 patient showing that myocardial tissue was probably injured by systemic cytokine release.
Myocarditis in COVID-19
Myocarditis can occur in all age groups of COVID-19 patients at an incidence of 19% to 28%, even when the patient has no previous history of cardiovascular disease, making it essential to identify the condition early. The diagnosis is based on abnormalities in the ECG findings, high troponin I values, or in Troponin T levels.
Patients who recovered from COVID-19 sometimes developed symptoms of cardiac injury.
Prognosis in COVID-19 With Cardiac Manifestations
Patients with COVID-19 who develop cardiac injury and inflammation typically have a poorer outcome, and experience complications during the course of their hospital stay. Cardiac injury increases the risk of mechanical ventilation, at 46%, well over tenfold the risk in patients with an intact heart. The incidence of ARDS is also higher, at almost 59% vs. 15% in patients without cardiac injury.
Acute kidney injury, electrolyte imbalance, and clotting disorders are all significantly higher in COVID-19 patients with a concomitant cardiac injury. Kidney injury is observed in over 8% in this subset vs. 0.3% of those without cardiac injury. Electrolyte imbalance is seen in 16% and clotting abnormalities in over 7%, compared to 5% and less than 2%, respectively.
Cardiac Arrhythmias
Patients with COVID-19 who developed cardiac injury also had a higher risk of dangerous arrhythmias, including ventricular tachycardia (VT) and ventricular fibrillation (VF), at 17% vs. 2%. The mortality rate is far higher, at over 50%, compared to less than 10%, among patients with and without cardiac injury.
Overall, arrhythmias were observed to occur in 17% to 44% of COVID-19 patients in various studies. Patients with higher TnT levels had a higher risk of malignant arrhythmias like VT and VF.
Biomarkers like CRP and D-dimer were elevated in patients with atrial tachyarrhythmias. Some drugs used for treating COVID-19, such as hydroxychloroquine and chloroquine, are known to cause VT or VF to occur by prolonging the QT interval. Thus, such drugs, especially hydroxychloroquine and azithromycin, are not to be administered in patients with a QT over 500 msec. All patients on these drugs should be monitored for daily QTc; potassium and magnesium levels should be kept above 4 and 2 mEq/L, respectively.
Prognosis
Patients with cardiac injury secondary to COVID-19 have a poor prognosis. Some prior research suggests that an increase in cardiac biomarkers like TnT, C-reactive protein (CRP), creatinine kinase myocardial band (CK-MB), lactate dehydrogenase (LDH), and creatinine kinase (CK).
Again, the impact of the COVID-19 pandemic extends beyond the direct or indirect cardiac injury. One study reported that patients with myocardial infarction received delayed care because of the extra precautions in procedures as routine as a 12-lead ECG, the overwhelm of emergency medical teams, delay on the part of patients in presenting for medical care due to the fear of contracting COVID-19 and having to test for the infection before necessary medical interventions. As a result, the course of the infarction tended to be more complicated, and the outcome worse.
Cardiac Arrest
Many studies have shown the occurrence of cardiac arrest among young adults with COVID-19. In the majority of cases, the event was secondary to hypoxia. The patient developed cardiac asystole in almost 90% of cases, with VT or VF in only 6%. Pulseless electrical activity (PEA) was seen in only 4% of patients. Only 13% of patients spontaneously resumed circulation. Of these, a meager four were still alive at 30 days, and only one retained functional neurological status at this time. The outcomes were better after VF or VT.
Coagulation Disorders
In a series of over 2,000 successive COVID-19 patients, 100 had signs of clinical decompensation. A quarter of them had a pulmonary embolism (PE). This figure was confirmed in another study. ICU patients with COVID-19 have twice the risk of PE compared to those in the ICU for other reasons.
The presence of PE increased the odds of requiring mechanical ventilation and ICU admission, of developing ARDS, disseminated intravascular coagulation, right ventricular failure, and cytokine storm. This latter refers to the severe systemic inflammation resulting from COVID-19 in some cases. It is thought to cause coagulation abnormalities via the action of interleukins and TNF-α. These cytokines are released from damaged endothelium and activated macrophages because of the hypoxia created by the lung injury.
The researchers say, “The conglomerate of an inflammatory storm, venous stasis from immobilization during hospitalization, and the hypercoagulability caused by treatment with glucocorticoids and immunoglobulins act in synergy to promote clot formation.” Moreover, clotting is promoted by the interleukins and complement proteins, causing vascular damage.
Markers of Coagulation Abnormalities
Some studies show that a high D-dimer level is linked to an elevated risk of venous thromboembolism (VTE). Moreover, both D-dimer and thrombocytopenia indicate a high platelet count and a poor prognosis. Several expert societies suggest that the presence of these markers should indicate the need for hospitalization due to the presence of high thrombin levels.
Such patients should receive thromboprophylaxis to improve the outcome. Monitoring of coagulation activity and treatment with anticoagulants is required. It is noted in some studies that even with standard anticoagulation, a fifth of critically ill patients went on to develop VTE, while a quarter had a hemorrhagic episode.
Patients who received both antivirals and direct oral anticoagulants (DOACs) had a marked rise in DOAC levels, leading to the withholding of antivirals from these COVID-19 patients until the antiviral was no longer being administered. If the antiviral was found essential, parenteral anticoagulants were to be used. However, the rates of hemorrhage and invasive mechanical ventilation were higher in patients on anticoagulation at standard doses vs. others in one study, but with no difference in mortality.
Stroke
COVID-19 patients with any of the following factors have a higher chance of developing a stroke, namely, older age, severe disease, hypertension, diabetes, and a marked elevation of inflammatory markers like CRP or D-dimer. The expression of the viral receptor ACE2 on the endothelium of the blood vessels, coupled with the binding of SARS-CoV-2 might increase the blood pressure. When this is operative along with the procoagulant status already described, and the presence of pro-inflammatory cytokines, the risk of a cerebrovascular event is high.
Again, impaired ACE3 function on the nerve cells along with reduced platelet counts and abnormal coagulation might disrupt normal autoregulatory mechanisms and cause hemorrhagic stroke.
The researchers point out, “Future studies are required to understand on a more mechanistic level the effect of COVID-19 on the myocardium and thus provide avenues to improve mortality and morbidity.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources